Topics
Matter in Our Surroundings
- Matter (Substance)
- Characteristics of Particles (Molecules) of Matter
- The Solid State
- The Liquid State
- The Gaseous State
- Plasma
- Bose-einstein Condensate
- Heat and change of physical state
- Concept of Evaporation
- Concept of Melting (Fusion)
- Concept of Boiling (Vaporization)
- Concept of Sublimation
- Concept of Freezing (Solidification)
- Concept of Condensation (Liquefaction)
- Concept of Desublimation (Deposition)
Is Matter Around Us Pure
- Matter (Substance)
- Natural substances
- Mixture
- Types of Mixtures
- Solution
- Concentration of a Solution
- Suspension Solution
- Colloidal Solution
- Evaporation Method
- Solvent Extraction (Using a Separating Funnel Method)
- Sublimation Method
- Chromatography Method
- Simple Distillation Method
- Fractional Distillation Method
- Crystallisation Method
- Classification of Change: Physical Changes
- Chemical Reaction
- Pure Substances
- Compound
- Elements
Atoms and Molecules
- History of Atom
- Laws of Chemical Combination
- Law of Conservation of Mass
- Law of Constant Proportions (Law of Definite Proportions)
- Dalton’s Atomic Theory
- Atoms: Building Blocks of Matter
- Symbols Used to Represent Atoms of Different Elements
- Atomic Mass
- Relative Atomic Mass (RAM)
- Molecules
- Classification of Molecules
- Difference Between Atoms and Molecules
- Ions (Radicals) and Its Types
- Chemical Formula or Molecular Formula
- Molecular Mass
- Formula Unit Mass
- Mole Concept
- Atoms and Molecules Numericals
Structure of the Atom
- Existence of Charged Particles in Matter
- Atoms: Building Blocks of Matter
- Discovery of Charged Particles in Matter
- Protons (p)
- Electrons (e)
- Neutrons (n)
- J. J. Thomson’s Atomic Model
- Advantage and Limitations of Thomson’s Atomic Model
- Lord Rutherford’s Atomic model
- Limitations of Rutherford’s Atomic Model
- Neils Bohr’s Model of an Atom
- Electronic Configuration of Atom
- Valency
- Different Ways to Determine Valency
- Atomic Number (Z), Mass Number (A), and Number of Neutrons (n)
- Atomic Mass
- Isotopes
- Uses of Radioactive Isotopes
- Isobars
- Atoms and Molecules Numericals
The Fundamental Unit of Life
- Cell: Structural and Functional Unit of Life
- The Invention of the Microscope and the Discovery of Cell
- Cell Theory
- Organisms Show Variety in Cell Number, Shape and Size
- Prokaryotic and Eukaryotic Cell
- Simple Diffusion
- Concept of Osmosis
- Osmotic Pressure
- Structure of the Cell
- Plasma Membrane
- Semi-permeable Membrane (Cell Membrane)
- Cell Wall - “Supporter and Protector”
- Nucleus - “Brain” of the Cell
- Cytoplasm - “Area of Movement”
- Endoplasmic Reticulum (ER)
- Golgi Apparatus - "The delivery system of the cell"
- Lysosome - “Suicidal Bag”
- Mitochondria - “Power House of the Cell”
- Plastids
- Non-living Substances Or Cell Inclusion
- Plant Cell and Animal Cell
- Cell Division: an Essential Life Process
Tissues
- Tissues - “The Teams of Workers”
- Plant and Animals Tissue
- Plant Tissues
- Meristems or Meristematic Tissues
- Permanent Tissue
- Simple Permanent Tissues (Supporting Tissue)
- Complex Permanent Tissues
- Complex Permanent Tissue: Xylem Structure and Function (Conducting Tissue)
- Complex Permanent Tissue: Phloem Structure and Function (Conducting Tissue)
- Animal Tissues
- Epithelial Tissue
- Connective Tissue
- Muscular Tissue
- Nervous Tissue
Motion
- Motion and Rest
- Describing Motion
- Motion Along a Straight Line
- Types of Motion
- Measuring the Rate of Motion - Speed with Direction
- Rate of Change of Velocity
- Distance and Displacement
- Displacement - Time Graph Or Distance - Time Graph
- Velocity - Time Graphs
- Equations of Motion by Graphical Method
- Derivation of Velocity - Time Relation by Graphical Method
- Derivation of Displacement - Time Relation by Graphical Method
- Derivation of Displacement - Velocity Relation by Graphical Method
- Uniform Circular Motion (UCM)
- Motion (Numerical)
Diversity in Living Organisms
- Biodiversity
- Biological Classification
- Classification of Living Organisms
- Taxonomic Hierarchy of Living Organisms: Unit of Classification
- Five Kingdom Classification
- Kingdom Monera
- Kingdom Protista
- Kingdom Fungi
- Classification of Kingdom Plantae
- Kingdom Animalia
- Differences Between Plantae (Plants) and Animalia (Animals)
- Classification of Kingdom Plantae
- Kingdom Plantae: Thallophyta (Algae)
- Kingdom Plantae: Thallophyta (Fungi)
- Division II- Bryophytes
- Division III- Pteridophytes
- Division I-Gymnosperms
- Division II- Angiosperms
- Kingdom Animalia
- Phylum: Porifera
- Phylum: Cnidaria/Coelenterata
- Phylum: Platyhelminthes
- Invertebrate: Phylum Nematoda
- Phylum: Annelida
- Phylum: Arthropoda
- Phylum: Mollusca
- Phylum: Echinodermata
- Subphylum: Prochordata
- Chordata: Vertebrata
- Invertebrata and Vertebrata
- Taxonomy and Systematics
- Nomenclature
Force and Laws of Motion
Gravitation
Work and Energy
Sound
- Sound
- Production of Sound
- Propagation of Sound
- Sound Need a Medium to Travel
- Sound Waves Are Longitudinal Waves
- Characteristics of a Sound Wave
- Speed of Sound (Velocity of Sound)
- Reflection of Sound
- Echoes
- Reverberation
- Uses of Multiple Reflection of Sound
- Range of Hearing in Humans
- Ultrasonic Sound Or Ultrasound
- SONAR
- Human Ear
- Sound (Numerical)
Improvement in Food Resources
- Improvements in Food Resources
- Improvement in Crop Yields
- Crop Variety Improvement
- Crop Production Improvement
- Crop Protection Management
- Methods to Replenish Nutrients in Your Soil
- Manuring (Biomanuring)
- Fertilizers
- Improved methods of agriculture
- Agricultural Assistance Programme
- Animal Husbandry (Livestock)
- Dairy Farming
- Poultry Farming
- Pisciculture (Fish Farming)
- Apiculture (Bee Farming)
Why Do We Fall ill
- Health
- Disease
- Categories of Disease
- Acute and Chronic Diseases
- Causes of Disease
- Communicable Or Infectious Diseases
- Infectious Agents
- Manifestation of Diseases
- Modes of Transmission of Diseases
- Organ-specific and Tissue-specific Manifestations
- Principles of Prevention of Diseases
- Principles of Treatment of Diseases
Natural Resources
- Natural Resources
- Biosphere: The Domain of Life
- Air is a Mixture
- Atmosphere and Its Layers
- Wind: The Movement of Air
- Rain
- Water: Our Lifeline
- Where Do We Get Water From?
- Availability of Water
- Importance of Water
- Water Pollution and Its Causes
- Mineral Riches in the Soil
- Biogeochemical Cycle
- Water Cycle
- Nitrogen Cycle
- The Carbon Cycle
- The Oxygen Cycle
- Ozone
- Ozone Layer Depletion
- Chemical formulae of compounds : A recapitulation
Notes
The chemical formula of a compound is a symbolic representation of its composition. The chemical formulae of different compounds can be written easily. For thisexercise, we need to learn the symbols and combining capacity of the lements.The combining power (or capacity) of an element is known as its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound. The valency of the atom of an element can be thought of as hands or arms of that atom.
Human beings have two arms and an octopus has eight. If one octopus has to catch hold of a few people in such a manner that all the eight arms of the octopus and both arms of all the humans are locked, how many humans do you hink the octopus can hold? Represent the octopus with O and humans with H. Can you write a formula for this combination? Do you get OH4 as the formula? The subscript 4 indicates the number of humans held by the octopus.
FORMULAE OF SIMPLE COMPOUNDS :
The simplest compounds, which are made up of two different elements are called binary compounds. Valencies of some ions are given in Table 3.6. You can use these to write formulae for compounds. While writing the chemical formulae for compounds, we write the constituent elements and their valencies as shown below. Then we must crossover the valencies of the combining atoms.
Examples :
1. Formula of hydrogen chloride
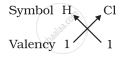
Formula of the compound would be HCl
2. Formula of hydrogen sulphide
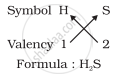
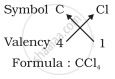
3. Formula of carbon tetrachloride
For magnesium chloride, we write the symbol of cation (Mg2+) first followed by the symbol of anion (Cl- ). Then their charges are criss-crossed to get the formula.
4. Formula of magnesium chloride
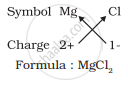
Thus, in magnesium chloride, there are two chloride ions (Cl- ) for each magnesium ion (Mg2+). The positive and negative charges must balance each other and the overall structure must be neutral. Note that in the formula, the charges on the ions are not indicated.
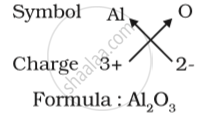
(a) Formula for aluminium oxide:
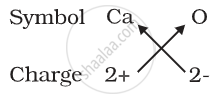
(b) Formula for calcium oxide:
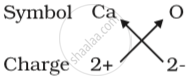
Here the valencies of the two elements are the same. you may arrive at the formula Ca2O2 But we simplify the formula as CaO.
(c) Formula of sodium nitrate:
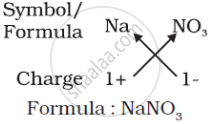
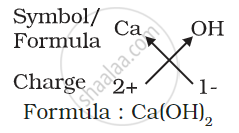
(d) Formula of calcium hydroxide:
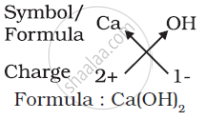
Note that the formula of calcium hydroxide is Ca(OH)2 and not CaOH2. We use brackets when we have two or more of the same ions in the formula. Here, the bracket around OH with a subscript 2 indicates that there are two hydroxyl (OH) groups joined to one calcium atom. In other words, there are two atoms each of oxygen and hydrogen in calcium hydroxide.
Notes
Formation of compounds:

Learn the above table very carefully for the ease of making chemical formulas
1) Formula for calcium oxide:
2) Formula of calcium hydroxide:
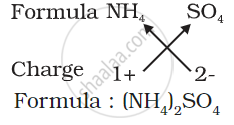
3) Formula of ammonium sulphate:
Note:
-
If the valencies of both elements are the same, they can cancel each other out and are simplified. Eg. It should be CaO and not Ca2O2.
-
In the case of polyatomic ions, the charge is designed to the entire group of ion. Eg. It should be Ca(OH)2 and not CaOH2 as the latter one means 2 atoms of hydrogen only!
