- Prepare sherbet by mixing water, sugar, lemon juice, and salt. Stir until the sugar dissolves.
- Prepare Bhel by mixing puffed rice, onions, tomatoes, coriander, spices, and sev in a bowl.
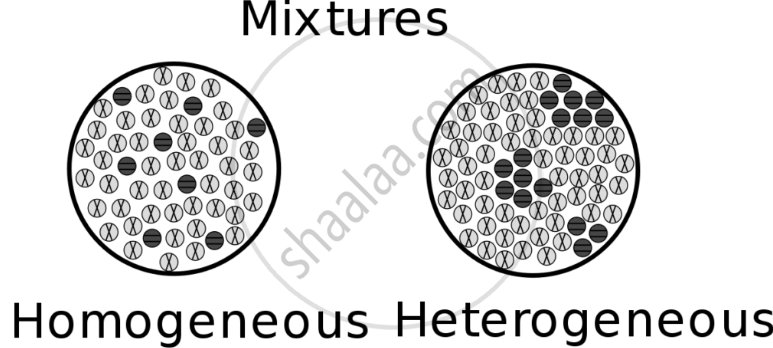
Observe:
- In sherbet, the sugar dissolves, but no new substance forms—it remains a mixture.
- In bhel, all ingredients stay separate and visible, showing that they do not chemically combine.
Conclusion:
Mixtures can be formed without a chemical change. The components of a mixture can be separated using simple methods like straining, sieving, or picking out ingredients. This shows that mixtures are different from chemical compounds, as their components retain their original properties.