Rutherford’s Gold Foil Experiment:
Rutherford used a very thin sheet of gold foil, only 0.0001 mm thick, and bombarded it with positively charged α-particles (alpha particles) emitted by a radioactive substance. He placed a fluorescent screen around the gold foil to observe how the α-particles behaved when they struck the foil.
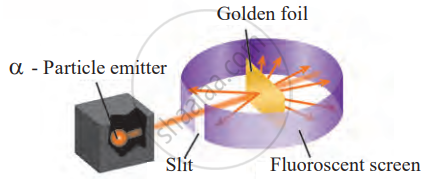
Rutherford’s scattering experiment
Observations,
- Most α-particles passed straight through: Rutherford observed that a majority of the α-particles went straight through the gold foil without any change in direction. This indicated that atoms have a lot of empty space inside.
- Some α-particles were detected slightly: A few α-particles were deflected at small angles. This suggested that they encountered a positively charged obstacle within the atom.
- A few α-particles deflected sharply or bounced back:A very small number of α-particles were deflected at large angles or even bounced back in the opposite direction. This surprising result indicated that the α-particles had collided with a dense, positively charged mass in the centre of the atom.
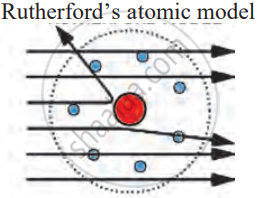
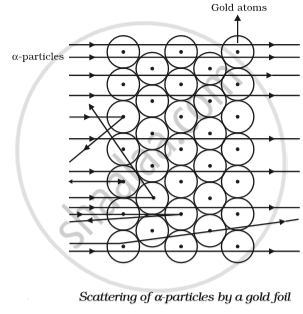
Following were his conclusions from the α-particle scattering experiment:
- Most of the space inside the atom is empty because most of the α-particles passed through the gold foil without getting deflected.
- Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
- A very small fraction of α-particles were deflected by 1800, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.




