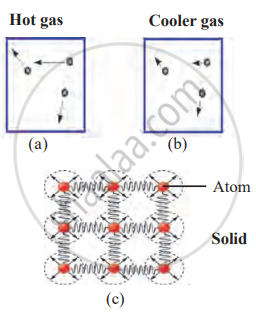
Temperature is measured in units of Celsius (°C), Fahrenheit (°F), and Kelvin (K). Kelvin is used in scientific experiments, while the other two are used in daily life. The relation between the three units is shown by the following formulas:
`"(F-32)" / "9"` = `"C" / "5"` ---------(1)Some specific temperatures are given in the three scales in the following table.
| Description | °F | °C | K |
| Boiling point of water | 212 | 100 | 373.15 |
| Freezing point of water | 32 | 0 | 273 |
| Room temperature | 72 | 23 | 296 |
| Boiling point of mercury | 674.06 | 356.7 | 629.85 |
| Freezing point of mercury | -38.8 | -39.33 | 233.82 |