Topics
Some Basic Concepts of Chemistry
- Importance and Scope of Chemistry
- Historical Approach to Particulate Nature of Matter
- Nature of Matter
- The International System of Units (SI)
- Concept of Mass and Weight
- Scientific Notation
- Significant Figures
- Dimensional Analysis
- Law of Conservation of Mass
- Law of Constant Proportions (Law of Definite Proportions)
- Law of Multiple Proportions
- Gay Lussac’s Law of Gaseous Volumes
- Avogadro's Law
- Dalton's Atomic Theory
- Atomic Mass
- Average Atomic Mass
- Molecular Mass
- Formula Mass
- Mole Concept
- Percentage Composition
- Stoichiometry and Stoichiometric Calculations - Introduction
- Limiting Reagent
- Concentration of a Solution
- Introduction of Some Basic Concepts of Chemistry
Structure of Atom
- Discovery of Electron
- Charge to Mass Ratio of Electron
- Charge on the Electron
- Discovery of Protons and Neutrons
- Atomic Model
- J. J. Thomson’s Atomic Model
- Lord Rutherford’s Atomic model
- Atomic Number (Z), Mass Number (A), and Number of Neutrons (n)
- Atomic Mass
- Isobars
- Isotopes
- Drawbacks of Rutherford Atomic Model
- Wave Nature of Electromagnetic Radiation
- Electromagnetic Waves : Numericals
- Particle Nature of Electromagnetic Radiation: Planck's Quantum Theory of Radiation
- Evidence for the Quantized Electronic Energy Levels - Atomic Spectra
- Bohr’s Model for Hydrogen Atom
- Dual Behaviour of Matter: De Broglie's relationship
- Heisenberg’s Uncertainty Principle
- Quantum Mechanical Model of Atom
- Quantum Mechanical Model of the Atom - Orbitals and Quantum Numbers
- Quantum Mechanical Model of the Atom - Concept of Shells and Subshells
- Quantum Mechanical Model of the Atom - Shapes of Atomic Orbitals
- Quantum Mechanical Model of the Atom - Energies of Orbitals
- Quantum Mechanical Model of the Atom - Filling of Orbitals in Atom
- Quantum Mechanical Model of the Atom - Electronic Configuration of Atoms
- Quantum Mechanical Model of the Atom - Stability of Completely Filled and Half Filled Subshells
- Structure of Atom Numericals
Classification of Elements and Periodicity in Properties
- Significance of Classification of Elements
- Genesis of Periodic Classification
- Modern Periodic Law and the Present Form of the Periodic Table
- Nomenclature of Elements with Atomic Number Greater than 100
- Periodic Table and Electronic Configuration
- The s-Block Elements
- The p-Block Elements
- The d-Block Elements (Transition Elements)
- The f-Block Elements (Inner-transition Elements)
- Metals, Non-metals and Metalloids
- Atomic Radius Or Atomic Size
- Ionic Radius
- Ionization Enthalpy or Ionization Energy (IE) or Ionization Potential (IP)
- Electron Gain Enthalpy
- Electronegativity
- Periodicity of Valence or Oxidation States
- Anomalous Properties of Second Period Elements
- Periodic Trends and Chemical Reactivity
- Classification of Elements and Periodicity in Properties Numericals
Chemical Bonding and Molecular Structure
- Kossel and Lewis Approach to Chemical Bonding
- Kossel-lewis Approach to Chemical Bonding - Octet Rule
- Kossel-lewis Approach to Chemical Bonding - Covalent Bond
- Lewis Structures (Lewis Representation of Simple Molecules)
- Kossel-lewis Approach to Chemical Bonding - Formal Charge
- Kossel-lewis Approach to Chemical Bonding - Limitations of the Octet Rule
- Ionic or Electrovalent Bond
- Bond Length
- Bond Angle
- Bond Enthalpy
- Bond Order
- Resonance Structures
- Polarity of Bonds
- Valence Shell Electron Pair Repulsion Theory (VSEPR)
- Valence Bond Theory
- Valence Bond Theory - Orbital Overlap Concept
- Valence Bond Theory - Directional Properties of Bonds
- Valence Bond Theory - Overlapping of Atomic Orbitals
- Valence Bond Theory - Types of Overlapping and Nature of Covalent Bonds
- Valence Bond Theory - Strength of Sigma (σ) bond and pi (π) bond
- Hybridisation - Introduction
- Types of Hybridisation
- Hybridisation of Elements Involving d Orbitals
- Molecular Orbital Theory - Introduction
- Formation of Molecular Orbitals - Linear Combination of Atomic Orbitals (LCAO)
- Conditions for the Combination of Atomic Orbitals
- Types of Molecular Orbitals
- Energy Level Diagram for Molecular Orbitals
- Electronic Configuration and Molecular Behaviour
- Bonding in Some Homonuclear Diatomic Molecules
- Hydrogen Bonding - Introduction
- Cause of Formation of Hydrogen Bond
- Types of Hydrogen Bonding
- Chemical Bonding and Molecular Structure Numericals
- States of Matter:- Gases and Liquids Numericals
States of Matter:- Gases and Liquids
- Intermolecular Forces - Introduction
- Dispersion Forces Or London Forces
- Dipole - Dipole Forces
- Dipole-induced Dipole Forces
- Hydrogen Bond
- Thermal Energy
- Intermolecular Forces Vs. Thermal Interactions
- The Gaseous State
- Boyle’s Law (Pressure - Volume Relationship)
- Charles’ Law (Temperature - Volume Relationship)
- Gay Lussac’s Law (Pressure- Temperature Relationship)
- Avogadro's Law
- Derivation of Ideal Gas Equation
- Density and Molar Mass of a Gaseous Substance
- Dalton’s Law of Partial Pressures
- Kinetic Molecular Theory of Gases
- Behaviour of Real Gases: Deviation from Ideal Gas Behaviour
- Liquefaction of Gases
- Vapour Pressure
- Surface Tension
- Viscosity
- States of Matter:- Gases and Liquids Numericals
Chemical Thermodynamics
- Thermodynamic Terms
- The State of the System
- The Internal Energy as a State Function - Work
- The Internal Energy as a State Function - Heat
- The Internal Energy as a State Function - the General Case
- Work
- Enthalpy, H - a Useful New State Function
- Enthalpy, H - Extensive and Intensive Properties
- Enthalpy, H - Heat Capacity
- Enthalpy, H - The Relationship Between Cp and Cv for an Ideal Gas
- Measurement of ∆U and ∆H Calorimetry - ∆U Measurements
- Measurement of ∆U and ∆H Calorimetry - ∆H Measurements
- Standard Enthalpy of Reactions
- Enthalpy Changes During Phase Transformations
- Standard Enthalpy of Formation
- Thermochemical Equations
- Hess’ Law of Constant Heat Summation
- Standard Enthalpy of Combustion
- Enthalpy of Atomization
- Bond Enthalpy
- Enthalpy of Solution
- Lattice Enthalpy
- Is Decrease in Enthalpy a Criterion for Spontaneity
- Entropy and Spontaneity
- Gibbs Energy and Spontaneity
- Entropy and Second Law of Thermodynamics
- Absolute Entropy and Third Law of Thermodynamics
- Gibbs Energy Change and Equilibrium
Equilibrium
- Concept of Equilibrium
- Solid-liquid Equilibrium
- Liquid-vapour Equilibrium
- Solid - Vapour Equilibrium
- Equilibrium Involving Dissolution of Solid in Liquids
- Equilibrium Involving Dissolution of Gases in Liquids
- General Characteristics of Equilibria Involving Physical Processes
- Equilibrium in Chemical Processes - Dynamic Equilibrium
- Law of Chemical Equilibrium and Equilibrium Constant
- Equilibrium Constant in Gaseous Systems
- Heterogeneous Equlibria
- Predicting the Extent of a Reaction
- Predicting the Direction of the Reaction
- Calculating Equilibrium Concentrations
- Relationship Between Equilibrium Constant K, Reaction Quotient Q and Gibbs Energy G
- Change of Concentration
- Change of Pressure
- Addition of Inert Gas
- Change of Temperature
- Effect of Catalyst
- Ionic Equilibrium in Solution
- Concept of Acid, Base, and Salt
- Arrhenius, Bronsted-lowry and Lewis Concept of Acids and Bases
- Concept of Ionization of Acids and Bases
- The Ionization Constant of Water and Its Ionic Product
- The pH Scale
- Ionization Constants of Weak Acids
- Ionization of Weak Bases
- Relation Between Ka and Kb
- Di- and Polybasic Acids and Di- and Polyacidic Bases
- Factors Affecting Acid Strength
- Common Ion Effect in the Ionization of Acids and Bases
- Hydrolysis of Salts and the Ph of Their Solutions
- Buffer Solutions
- Concept of Solubility Equilibria of Sparingly Soluble Salts
Redox Reactions
- Classical Idea of Redox Reactions - Oxidation and Reduction Reactions
- Redox Reactions in Terms of Electron Transfer Reactions - Introduction
- Redox Reactions in Terms of Electron Transfer Reactions - Competitive Electron Transfer Reactions
- Oxidation Number - Introduction
- Types of Redox Reactions
- Balancing Redox Reactions in Terms of Loss and Gain of Electrons
- Redox Reactions as the Basis for Titrations
- Limitations of Concept of Oxidation Number
- Redox Reactions and Electrode Processes
Organic Chemistry - Some Basic Principles and Techniques
- Tetravalence of Carbon - Shapes of Organic Compounds
- Complete, Condensed and Bond-line Structural Formulas
- Three-dimensional Representation of Organic Molecules
- Classification of Organic Compounds
- The IUPAC System of Nomenclature
- IUPAC Nomenclature of Alkanes
- Nomenclature of Organic Compounds Having Functional Group(s)
- Nomenclature of Substituted Benzene Compounds
- Isomerism
- Fission of a Covalent Bond
- Nucleophiles and Electrophiles
- Electron Movement in Organic Reactions
- Electron Displacement Effects in Covalent Bonds
- Inductive Effect
- Resonance Structure
- Resonance Effect
- Electromeric Effect (E Effect)
- Hyperconjugation
- Types of Organic Reactions and Mechanisms
- Introduction of Methods of Purification of Organic Compounds
- Sublimation Method
- Crystallisation Method
- Simple Distillation Method
- Solvent Extraction (Using a Separating Funnel Method)
- Chromatography Method
- Qualitative Analysis of Organic Compounds - Detection of Carbon and Hydrogen
- Qualitative Analysis of Organic Compounds - Detection of Other Elements
- Quantitative Analysis of Carbon and Hydrogen
- Quantitative Analysis of Nitrogen
- Quantitative Analysis of Halogens
- Quantitative Analysis of Sulphur
- Quantitative Analysis of Phosphorus
- Quantitative Analysis of Oxygen
Hydrocarbons
- Classification of Hydrocarbons
- Alkanes - Introduction
- Nomenclature and Isomerism
- Preparation of Alkanes from Unsaturated Hydrocarbons, Alkyl Halides and Carboxylic Acids
- Physical Properties of Alkanes
- Chemical Properties of Alkanes
- Conformations (Ethane)
- Alkenes - Introduction
- Structure of Double Bond (Ethene)
- Nomenclature
- Isomerism
- Preparation of Alkenes from Alkynes, Alkyl Halides, Vicinal Dihalides and Alcohols by Acidic Dehydration
- Physical Properties of Alkenes
- Chemical Properties of Alkenes
- Alkynes - Introduction
- Nomenclature and Isomerism
- Structure of Triple Bond
- Preparation of Alkynes from Calcium Carbide and Vicinal Dihalides
- Physical Properties of Alkynes
- Chemical Properties of Alkynes
- Aromatic Hydrocarbons
- Nomenclature and Isomerism
- Structure of Benzene
- Aromaticity (Huckel Rule)
- Preparation of Benzene
- Physical Properties of Aromatic Hydrocarbons
- Chemical Properties of Aromatic Hydrocarbons
- Electrophilic Substitution Reactions
- Mechanism of Electrophilic Substitution Reactions
- Directive Influence of a Functional Group in Monosubstituted Benzene
- Carcinogenicity and Toxicity
Hydrogen
- Position of Hydrogen in the Periodic Table
- Dihydrogen
- Preparation of Dihydrogen
- Properties and Uses of Dihydrogen
- Ionic or Saline Hydrides
- Covalent or Molecular Hydride
- Metallic or Non-stoichiometric (or Interstitial) Hydrides
- Physical Properties of Water
- Structure of Water
- Structure of Ice
- Chemical Properties of Water
- Classification of water: Soft and Hard Water
- Temporary Hardness of Water
- Permanent Hardness of Water
- Preparation of Hydrogen Peroxide
- Physical Properties of Hydrogen Peroxide
- Structure of Hydrogen Peroxide
- Chemical Properties of Hydrogen Peroxide
- Storage of Hydrogen Peroxide
- Uses of Hydrogen Peroxide
- Heavy Water
- Dihydrogen as a Fuel
S-block Elements (Alkali and Alkaline Earth Metals)
- Group 1 Elements - Alkali Metals
- General Characteristics of the Compounds of the Alkali Metals
- Anomalous Properties of Lithium
- Some Important Compounds of Sodium
- Biological Importance of Sodium and Potassium
- Group 2 Elements - Alkaline Earth Metals
- General Characteristics of the Compounds of the Alkaline Earth Metals
- Anomalous Behaviour of Beryllium
- Some Important Compounds of Calcium
- Biological Importance of Magnesium and Calcium
Some P-block Elements
- Introduction to p-block Elements
- Group 13 Elements - The Boron Family
- Important Trends and Anomalous Properties of Boron
- Some Important Compounds of Boron
- Uses of Boron and Aluminium and Their Compounds
- Group 14 Elements - The Carbon Family
- Important Trends and Anomalous Behaviour of Carbon
- Allotropes of Carbon - Diamond
- Allotropes of Carbon - Graphite
- Allotropes of Carbon - Fullerenes
- Allotropes of Carbon - Uses of Carbon
- Some Important Compounds of Carbon and Silicon - Carbon Monoxide
- Some Important Compounds of Carbon and Silicon - Carbon Dioxide
- Some Important Compounds of Carbon and Silicon - Silicon Dioxide
- Some Important Compounds of Carbon and Silicon - Silicones
- Some Important Compounds of Carbon and Silicon - Silicates
- Some Important Compounds of Carbon and Silicon - Zeolites
Environmental Chemistry
- Environmental Pollution - Introduction
- Tropospheric Pollution - Gaseous Air Pollutants
- Tropospheric Pollution - Particulate Pollutants
- Stratospheric Pollution
- Water Pollution and Its Causes
- International Standards for Drinking Water
- Soil Pollution - Pesticides, Herbicides
- Industrial Waste
- Strategies to Control Environmental Pollution
- Green Chemistry - Introduction
- Green Chemistry in Day-to-day Life
- Discovery of Electrons
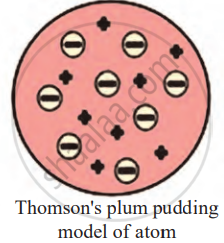
- Thomson’s Plum Pudding Model of the Atom
- Merits and Demerits
Discovery of Electrons:
J.J. Thomson, a renowned scientist, conducted experiments and discovered that atoms contain negatively charged particles called electrons. He showed that these electrons are extremely light, with a mass about 1800 times smaller than a hydrogen atom, which is the lightest atom known. Despite having negatively charged electrons, most substances are electrically neutral. This means that the overall charge of an atom must balance out to zero.

Thomson’s Plum Pudding Model of the Atom:
To explain why atoms are neutral even though they contain negatively charged electrons, Thomson proposed a model called the Plum Pudding Model in 1904.
- Thomson proposed the comparison of an atom to a pudding or a sphere of positive charge.
- In this sphere of positive charge, the negatively charged electrons are scattered throughout, similar to plums embedded in a pudding.
- The positive charge is spread evenly throughout the atom, and the negative charges (electrons) are embedded within it.
- The positive and negative charges balance each other out, making the atom electrically neutral overall.
Thomson proposed that,
- An atom consists of a positively charged sphere, and the electrons are embedded in it.
- The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.

Merits and Demerits:
| Merits | Demerits |
| Thomson's model introduced electrons as negatively charged particles within the atom. | Thomson's model couldn’t explain the stability of the atom or how electrons were arranged. |
| It explained that atoms are electrically neutral by balancing negative electrons with a positive charge distributed uniformly. | It did not recognise the presence of a dense, central nucleus, discovered later by Rutherford. |
| Provided a stepping stone for further research on the internal structure of atoms. | The model failed to explain the gold foil experiment, which revealed that most of the atom is empty space. |
If you would like to contribute notes or other learning material, please submit them using the button below.
Shaalaa.com | Dalton Atomic Theory and Thompson Model
to track your progress
