Advertisements
Advertisements
Question
Complete the following radioactive reaction:
\[\ce{^-_-X -> ^-_-Y + ^4_2He -> ^234_91Z + ^0_-1e}\]
Solution
\[\ce{^\underline{238}_\underline{92}X -> ^\underline{234}_\underline{90}Y + ^4_2He -> ^234_91Z + ^0_-1e}\]
APPEARS IN
RELATED QUESTIONS
Represent the change in the nucleus of a radioactive element when a β particle is emitted.
Radiations given out from a source when subjected to an electric field in a direction perpendicular to their path are shown below in the diagram. The arrows show the path of the radiation A, B and C. Answer the following questions in terms of A, B and C.
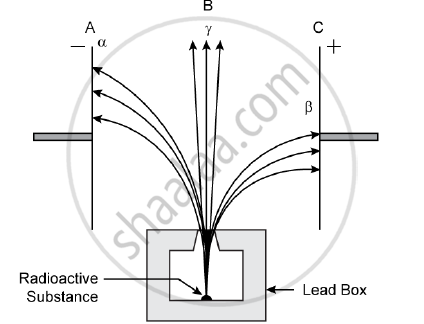
1) Name the radiation B which is unaffected by the electrostatic field.
2) Why does the radiation C deflect more than A?
3) Which among the three causes the least biological damage externally.
4) Name the radiation which is used in carbon dating.
What do you mean by Atomic number
What happens to the atomic number of element when (i) An α -particle, (ii) A β -particle 1 and (iii) γ-radiation is emitted?
Name two radioactive substances.
Define bound electrons.
What is meant by Radioactivity?
From α, β and γ-rays, name the one which travels with the speed of light?
Unit of radioactivity is _______
