Advertisements
Advertisements
Question
Complete the reaction mentioning all the products formed:
\[\ce{2KMnO4 ->[\Delta]}\]
Solution
\[\ce{2KMnO4 ->[\Delta] K2MnO4 + MnO2 + O2}\]
APPEARS IN
RELATED QUESTIONS
Complete the following equations:
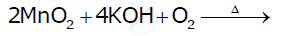
Complete the following equation:
\[\ce{2MnO4- + 6H+ + 5NO2- ->}\]
When chromite ore FeCr2O4 is fused with NaOH in presence of air, a yellow-coloured compound (A) is obtained, which on acidification with dilute sulphuric acid gives a compound (B). Compound (B) on reaction with KCl forms an orange coloured crystalline compound (C).
(i) Write the formulae of the compounds (A), (B) and C.
(ii) Write one use of compound (C).
Complete the following equation : MnO4- + 8H+ + 5e- →
Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with iron (II) ions? Write the ionic equation for the reaction.
Zinc carbonate is precipitated from zinc sulphate solution by the addition of ___________.
When \[\ce{Cu^2+}\] ion is treated with \[\ce{KI}\], a white precipitate is formed. Explain the reaction with the help of chemical equation.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
Indicate the steps in the preparation of \[\ce{K2Cr2O7}\] from chromite ore.
