Advertisements
Advertisements
Question
Consider the following compounds:
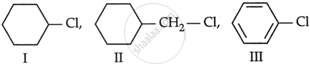
The correct order of reactivity towards SN2 reaction
Options
I > III > II
II > III > I
II > I > III
III > I > II
MCQ
Solution
II > I > III
Explanation:
Chlorine's I effect attracts electrons, resulting in a tiny positive charge on the carbon bonded.
Compound 2 has the strongest I effect of chlorine, rendering it susceptible to the SN2 reaction.
In compound 3, the strong I impact of chlorine is balanced by the resonance effect, allowing replacement at the o- and p- positions.
Compound 1 balances the negative effect of chlorine with a positive effect of the cycloalkyl group.
shaalaa.com
Is there an error in this question or solution?
