Science (English Medium)
Academic Year: 2021-2022
Date & Time: 14th December 2021, 11:30 am
Duration: 1h30m
Advertisements
General Instructions:
- This question paper contains 55 questions out of which 45 questions are to be attempted.
- All questions carry equal marks.
- This question paper consists of three Sections - Section A, B and C.
- Section A contains 25 questions. Attempt any 20 questions from Q. No. 01 to 25.
- Section B contains 24 questions. Attempt any 20 questions from Q. No. 26 to 49.
- Section C contains 6 questions. Attempt any 5 questions from Q. No. 50 to 55.
- The first 20 questions attempted in Section A and Section B and first 5 questions attempted in Section C by a candidate will be evaluated.
- There is only one correct option for every multiple choice questions (MCQ). Marks will not be awarded for answering more than one option.
- There is no negative marking.
Which one of the following pairs will form an ideal solution?
chloroform and acetone
ethanol and acetone
n-hexane and n-heptane
phenol and aniline
Chapter:
Which of the following is known as amorphous solid?
Glass
Plastic
Rubber
All of the above
Chapter:
The
slightly greater than 109°28'
slightly less than 109°28'
slightly greater than 120°
slightly less than 120°
Chapter:
Consider the following reaction
Chapter:
Nucleoside are composed of ______.
a pentose sugar and phosphoric acid.
a nitrogenous base and phosphoric acid.
a nitrogenous base and a pentose sugar.
a nitrogenous base, a pentose sugar and phosphoric acid.
Chapter:
The oxidation state −2 is most stable in ______.
O
S
Se
Te
Chapter:
Which of the following is not a characteristic of a crystalline solid?
Definite and characteristic heat of fusion.
Isotropic nature.
A regular periodically repeated pattern of arrangement of constituent particles in the entire crystal.
A true solid.
A regular arrangement of constituent particles.
Sharp melting point.
Chapter: [0.01] Solid State
Which of the following formula represents Raoult's law for a solution containing non-volatile solute?
Chapter:
An azeotropic solution of two liquids has a boiling point lower than either of the two when it ______.
shows a positive deviation from Raoult's law.
shows a negative deviation from Raoult's law.
shows no deviation from Raoult's law.
is saturated.
Chapter:
Which of the following crystal will show metal excess defect due to extra cation?
Chapter:
Which of the following acids reacts with acetic anhydride to form a compound Aspirin?
Benzoic acid
Salicylic acid
Phthalic acid
Acetic acid
Chapter:
Which of the following statements is wrong?
Oxygen shows pπ-pπ bonding.
Sulphur shows little tendency of catenation.
Oxygen is diatomic whereas sulphur is polyatomic.
Chapter:
Amino acids which cannot be synthesized in the body and must be obtained through diet are known as ______.
Acidic amino acids
Essential amino acids
Basic amino acids
Non-essential amino acids
Chapter:
Which one of the following halides contains
Allyl halide
Alkyl halide
Benzyl halide
Vinyl halide
Chapter:
On mixing 20 ml of acetone with 30 ml of chloroform. The total volume of the solution is ______.
< 50 ml
= 50 ml
> 50 ml
= 10 ml
Chapter:
Consider the following compounds:
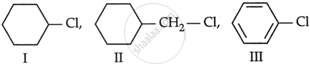
The correct order of reactivity towards SN2 reaction
I > III > II
II > III > I
II > I > III
III > I > II
Chapter:
Which of the following forms strong pπ - pπ bonding?
Chapter:
Advertisements
low
low
high
low
Chapter:
Which of the following sugar is known as dextrose?
Glucose
Fructose
Ribose
Sucrose
Chapter:
N2O
NO2
NO
N2
Chapter:
Which of the following is a network solid?
SO2
SiO2
CO2
H2O
Chapter:
Major product formed in the following reaction:
Chapter:
Chlorine reacts with cold and dilute
NaCl and NaClO3
NaCl and NaClO
NaCl and NaClO4
NaClO and NaClO3
Chapter:
Elevation of boiling point is inversely proportional to ______.
molal elevation constant (Kb)
molality (m)
molar mass of solute (M)
weight of solute (W)
Chapter:
An unknown gas 'X' is dissolved in water at 2.5 bar pressure and has mole fraction 0.04 in solution. The mole fraction of 'X' gas when the pressure of gas is doubled at the same temperature is ______.
0.08
0.04
0.02
0.92
Chapter:
The base which is present in DNA but not in RNA.
Cytosine
Guanine
Adenine
Thymine
Chapter:
In the following reaction
CH3 − CHO and CH3CH2OH
CH3 − CH = CH − COOH
CH3 − CH = CH − CHO
CH3 − CH2 − CH2 − CHO
Chapter:
Enantiomers differ only in ______.
boiling point
rotation of polarized light
melting point
solubility
Chapter:
The number of lone pairs of electrons in
zero
one
two
three
Chapter:
Sulphuric acid is used to prepare more volatile acids from their corresponding salts due to its ______.
strong acidic nature
low volatility
strong affinity for water
ability to act as a dehydrating agent
Chapter:
An element with density 6 g cm−3 forms a fcc lattice with edge length of 4 × 10−8 cm. The molar mass of the element is ______.
(NA = 6 × 1023 mol−1)
57.6 g mol−1
28.8 g mol−1
82.6 g mol−1
62 g mol−1
Chapter:
Which of the following is the weakest reducing agent in group 15?
Chapter:
The boiling point of a 0.2 m solution of a non-electrolyte in water is ______.
(Kb, for water = 0.52 K kg mol−1)
100°C
100.52°C
100.104°C
l00.26°C
Chapter:
Nucleic acids are polymer of ______.
amino acids
nucleosides
nucleotides
glucose
Chapter:
Advertisements
Which of the following gas dimerises to become stable?
Chapter:
In the following diagram point, 'X' represents
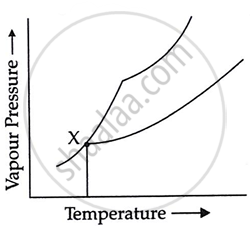
Boiling point of solution
Freezing point of solvent
Boiling point of solvent
Freezing point of solution
Chapter:
XeF6 on reaction with NaF gives ______.
Na+[XeF7]-
[NaF2]-[XeF5]+]
Na+[XeF6]-
[NaF2]+[XeF5]-
Chapter:
Glucose on reaction with
Saccharic acid
Hexanoic acid
Gluconic acid
Salicylic acid
Chapter:
Which of the following is optically inactive?
(+)−Butan-2-ol
(−)−Butan-2-ol
(±)−Butan-2-ol
(+)−2-Bromobutane
Chapter:
Which of the following is not a correct statement?
Halogens are strong oxidising agents.
Halogens are more reactive than interhalogens.
All halogens are coloured.
Halogens have maximum negative electron gain enthalpy.
Chapter:
Which of the following has highest boiling point?
C2H5 − F
C2H5 − Cl
C2H5 − Br
C2H5 − I
Chapter:
Which of the following isomer of pentane C5H12 will give three isomeric monochlorides on photochemical chlorination?
CH3CH2CH2CH2CH3
All of the above
Chapter:
Assertion (A): A raw mango placed in a saline solution loses water and shrivel into pickle.
Reason (R): Through the process of reverse osmosis, raw mango shrivel into pickle.
Both A and R are true and R is the correct explanation of A.
Both A and R are true but R is not the correct explanation of A.
A is true but R is false.
A is false but R is true.
Chapter:
Assertion (A): H2S is less acidic than H2Te.
Reason (R): H-S bond has more Δbond H° than H-Te bond.
Both A and R are true and R is the correct explanation of A.
Both A and R are true but R is not the correct explanation of A.
A is true but R is false.
A is false but R is true.
Chapter:
Assertion (A): Chlorobenzene is less reactive towards nucleophilic substitution reaction.
Reason (R): Nitro group in chlorobenzene increases its reactivity towards nucleophilic substitution reaction.
Both A and R are true and R is the correct explanation of A.
Both A and R are true but R is not the correct explanation of A.
A is true but R is false.
A is false but R is true.
Chapter:
Assertion (A): Due to schottky defect, there is no effect on the density of a solid.
Reason (R): Equal number of cations and anions are missing from their normal sites in Schottky defect.
Both A and R are true and R is the correct explanation of A.
Both A and R are true but R is not the correct explanation of A.
A is true but R is false.
A is false but R is true.
Chapter:
Assertion (A): Fluorine forms only one oxoacid HOF.
Reason (R): Fluorine atom is highly electronegative.
Both A and R are true and R is the correct explanation of A.
Both A and R are true but R is not the correct explanation of A.
A is true but R is false.
A is false but R is true.
Chapter:
Match the following:
| I | II | ||
| (i) | Stoichiometric defects | (a) | Crystalline solids |
| (ii) | Long range order | (b) | F-centres |
| (iii) | ...ABC ABC ABC... | (c) | Schottky and Frenkel defects |
| (iv) | Number of atoms per unit cell = 2 | (d) | fcc structure |
| (v) | Metal excess defect due to anionic vacancies |
Which of the following is the best matched options?
(i) - (d), (ii) - (a), (iii) - (b), (iv) - (c)
(i) - (c), (ii) - (a), (iii) - (d), (v) - (b)
(i) - (c), (ii) - (a), (iii) - (d), (iv) - (b)
(i) - (a), (ii) - (b), (v) - (c), (iv) - (d)
Chapter:
Which of the following analogies is correct?
XeF2 : linear :: XeF6 : square planar
moist SO2 : Reducing agent :: Cl2 : bleaching agent
N2 : Highly reactive gas :: F2 : inert at room temperature
NH3 : strong base :: HI : weak acid
Chapter:
Complete the following analogy:
Curdling of milk : A :: α-helix : B
A: Primary structure
B: Secondary structure
A: Denatured protein
B: Primary structure
A: Secondary structure
B: Denatured protein
A: Denatured protein
B: Secondary structure
Chapter:
Read the passage given below and answer the following question:
| Alcohols and phenols are acidic in nature. Electron withdrawing groups in phenol increase its acidic strength and electron donating groups decrease. Alcohols undergo nucleophilic substitution with hydrogen halides to give alkyl halides. On oxidation primary alcohols yield aldehydes with mild oxidising agents and carboxylic acids with strong oxidising agents while secondary alcohols yield ketones. The presence of -OH groups in phenols activates the ring towards electrophilic substitution. Various important products are obtained from phenol like salicylaldehyde, salicylic acid, picric acid, etc. |
Which of the following alcohols is resistant to oxidation?
Chapter:
Read the passage given below and answer the following question:
| Alcohols and phenols are acidic in nature. Electron withdrawing groups in phenol increase its acidic strength and electron donating groups decrease. Alcohols undergo nucleophilic substitution with hydrogen halides to give alkyl halides. On oxidation primary alcohols yield aldehydes with mild oxidising agents and carboxylic acids with strong oxidising agents while secondary alcohols yield ketones. The presence of |
Which of the following group increases the acidic character of phenol?
CH3O-
All of these
Chapter:
Read the passage given below and answer the following question:
| Alcohols and phenols are acidic in nature. Electron withdrawing groups in phenol increase its acidic strength and electron donating groups decrease. Alcohols undergo nucleophilic substitution with hydrogen halides to give alkyl halides. On oxidation primary alcohols yield aldehydes with mild oxidising agents and carboxylic acids with strong oxidising agents while secondary alcohols yield ketones. The presence of −OH groups in phenols activates the ring towards electrophilic substitution. Various important products are obtained from phenol like salicylaldehyde, salicylic acid, picric acid, etc. |
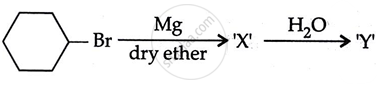
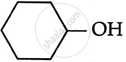
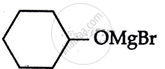
Consider the following reaction:
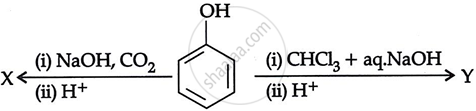
the products X and Y are
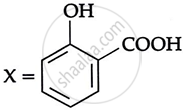 |
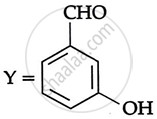 |
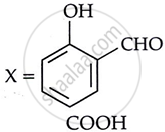 |
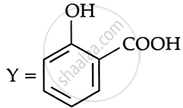 |
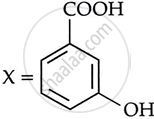 |
 |
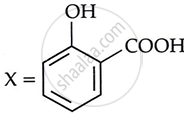 |
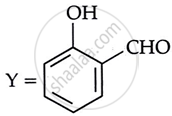 |
Chapter:
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2021 - 2022
Previous year Question paper for CBSE Class 12 -2022 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.