Advertisements
Advertisements
Question
Elevation of boiling point is inversely proportional to ______.
Options
molal elevation constant (Kb)
molality (m)
molar mass of solute (M)
weight of solute (W)
MCQ
Fill in the Blanks
Solution
Elevation of boiling point is inversely proportional to molar mass of solute (M).
Explanation:
Boiling Point Elevation
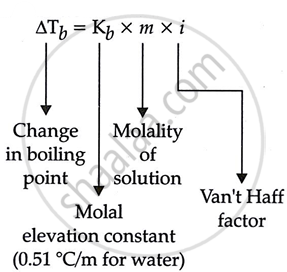
Molality `m = x/W xx 1000` ...(i)
Where, Molality = m
Number of mole of solute = x
Weight of solvent in grams = W ...(ii)
Moles of solute = mass of solute/molar mass of solute ...(iii)
From eq. (i), (ii), (iii), we derive that elevation of boiling point is inversely proportional to the molar mass of the solute.
shaalaa.com
Is there an error in this question or solution?
