Advertisements
Advertisements
Question
Draw a diagram showing the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules.
Solution
C2H4 :
The electronic configuration of C-atom in the excited state is:
`""_6"C" = 1"s"^2 2"s"^1 2"p"_x^1 2"p"_"y"^1 2"p"_"z"^1`
In the formation of an ethane molecule (C2H4), one sp2 hybrid orbital of carbon overlaps a sp2 hybridized orbital of another carbon atom, thereby forming a C-C sigma bond.
The remaining two sp2 orbitals of each carbon atom form a sp2-s sigma bond with two hydrogen atoms. The unhybridized orbital of one carbon atom undergoes sidewise overlap with the orbital of a similar kind present on another carbon atom to form a weak π-bond.
(a)

(b)

(c)
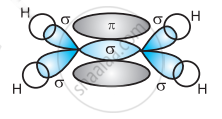
(d)

C2H2 :
In the formation of C2H2 molecule, each C–atom is sp hybridized with two 2p-orbitals in an unhybridized state.
One sp orbital of each carbon atom overlaps with the other along the internuclear axis forming a C–C sigma bond. The second sp orbital of each C–atom overlaps a half-filled 1s-orbital to form a σ bond.
The two unhybridized 2p-orbitals of the first carbon undergo sidewise overlap with the 2p orbital of another carbon atom, thereby forming two pi (π) bonds between carbon atoms. Hence, the triple bond between two carbon atoms is made up of one sigma and two π-bonds.
(a)
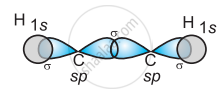
(b)
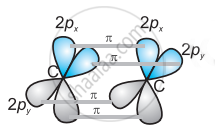
(c)
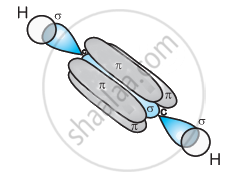
APPEARS IN
RELATED QUESTIONS
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar?
What is the total number of sigma and pi bonds in the following molecules?
C2H2
What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
What is the total number of sigma and pi bonds in the following molecules?
C2H4
Which of the following angle corresponds to sp2 hybridisation?
Match the shape of molecules in Column I with the type of hybridisation in Column II.
| Column I | Column II |
| (i) Tetrahedral | (a) sp2 |
| (ii) Trigonal | (b) sp |
| (iii) Linear | (c) sp3 |
Discuss the concept of hybridisation. What are its different types in a carbon atom.
What is the type of hybridisation of carbon atoms marked with star.
\[\begin{array}{cc}
\phantom{.....}\ce{O}\\
\phantom{.....}||\\
\ce{\overset{∗}{C}H2 = CH - \overset{∗}{C} - O - H}
\end{array}\]
What is the type of hybridisation of carbon atoms marked with star.
\[\begin{array}{cc}
\phantom{..........}\ce{O}\\
\phantom{..........}||\\
\ce{CH3 - CH2 - \overset{∗}{C} - H}
\end{array}\]
What is the type of hybridisation of carbon atoms marked with star.
\[\ce{\overset{∗}{C}H3 - CH = CH - CH3}\]
What is the type of hybridisation of carbon atoms marked with star.
\[\ce{CH3 - \overset{∗}{C} ≡ CH}\]
BF3 is a planar and electron-deficient compound. Hybridization and the number of electrons around the central atom, respectively are ______.
In the given reaction,
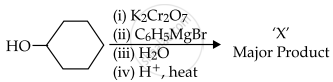
the number of sp2 hybridised carbon (s) in compound 'X' is ______.
In which of the following species S atom assumes sp3 hybrid state?
(I) (SO3)
(II) SO2
(III) H2S
(IV) S8
The hybridisation of carbanion is:
