Advertisements
Advertisements
Question
Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
\[\ce{BF3 + NH3 → F3B.NH3}\]
Solution 1
Boron atom in BF3 is sp2 hybridized. The orbital picture of boron in the excited state can be shown as:
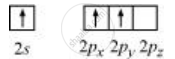
Nitrogen atom in NH3 is sp3 hybridized. The orbital picture of nitrogen can be represented as:

After the reaction has occurred, an adduct F3B⋅NH3 is formed as hybridization of ‘B’ changes to sp3. However, the hybridization of ‘N’ remains intact.
Solution 2
In BF3, B atom is sp2 hybridised. In NH3, N is sp3 hybridised. After the reaction, hybridisation of B changes from sp2 to sp3.
APPEARS IN
RELATED QUESTIONS
What is meant by hybridisation of atomic orbitals?
Describe the shapes of sp, sp2,sp3 hybrid orbitals.
Describe the change in hybridisation (if any) of the Al atom in the following reaction.
\[\ce{AlCl_3 + Cl- -> AlCl_4-}\]
Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why? (a) 1s and 1s (b) 1s and 2px (c) 2py and 2py (d) 1s and 2s.
Which hybrid orbitals are used by carbon atoms in the following molecules?
CH3–CH3
Which hybrid orbitals are used by carbon atoms in the following molecules?
CH3–CH=CH2
Which hybrid orbitals are used by carbon atoms in the following molecules?
CH3-CH2-OH
Which hybrid orbitals are used by carbon atoms in the following molecules?
CH3-CHO
Which hybrid orbitals are used by carbon atoms in the following molecules?
CH3COOH
The number of electron pairs involved in the formation · of hydrogen cyanide molecule are
AB3 is an interhalogen T-shaped molecule. The number of lone pairs of electrons on A is ______. (Integer answer)
