Advertisements
Advertisements
Question
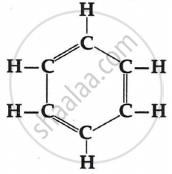
Draw the structures of possible isomers of butane, C4H10.
Solution
Isomers of Butane: There are two isomers. N-butane and iso-butane

APPEARS IN
RELATED QUESTIONS
Write the number of covalent bonds in the molecule of propane, C3H8.
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
if A is a cyclic compound
What is the molecular formula and structural formula of a cyclic hydrocarbon whose one molecule contains 8 hydrogen atoms?
Three organic compounds A, B and C have the following molecular formulae: C4H8O
Which molecular formula can represent an aldehyde as well as a ketone? Write the names and structural formulae of the aldehyde and ketone represented by this molecular formula.
Pentane has the molecular formula C5H12. It has ______.
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
Write the chemical equation for the following:
Hydrogenation of ethene
Draw the structure of propanol.
Name the following:
Draw the structure of propanone.
