Advertisements
Advertisements
Question
‘E’ is an element which reacts with oxygen to form an oxide E2O. An aqueous solution of E2O turns red litmus blue, so:
i. What is the nature of oxide E2O ?
ii. Write the name of element ‘E’.
Solution
(i) The nature of oxide E2O is basic.
(ii) The name of the element 'E' is sodium.
RELATED QUESTIONS
Metal A has electronic configuration (2, 8, 1) and metal B has (2, 8, 8, 2). Which is more reactive? Why? Identify these metals.
Write the chemical reaction when iron reacts with dilute H2SO4.
How would you show that silver is chemically less reactive than copper?
Give reason for the following:
Blue colour of copper sulphate solution is destroyed when iron filings are added to it.
Complete and balance the following equation:
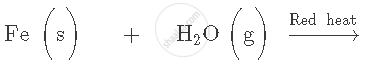
You are given samples of three metals − sodium, magnesium and copper. Suggest any two activities to arrange them in order of their decreasing reactivities.
The elements whose oxides can turn phenolphthalein solution pink are:
(a) Na and K
(b) K and C
(c) Na and S
(d) K and P
What is the characteristic of the electronic configuration of noble gases?
Answer the following question:
An element X on reacting with oxygen forms an oxide X2O. This oxide dissolves in water and turns red litmus blue. State whether element X is metal or a non - metal. Explain with a proper example.
Why is acidified water considered to be a good conductor of electricity?
