Advertisements
Advertisements
Question
Explain, all alkalis are bases but all bases are not alkalis.
Solution
An alkali is a base which is soluble in water but all bases are not water soluble.
For example Ferric hydroxide [Fe(OH)3] and cupric hydroxide [ Cu(OH)2 ] are bases but these are not soluble in water but sodium hydroxide NaOH, calcium hydroxide Ca(OH)2 are bases and are also soluble in water.
Hence it is rightly said that all alkalis are bases but all bases are not alkalis.
Concept Insight : An alkali is a basic hydrooxide which when dissolved in water produces hydroxyl (OH-) ions as the only negatively charged ions.
Example : NaOH(aq) ⇌ Na+ + OH-
APPEARS IN
RELATED QUESTIONS
Dorji has a few bottles of soft drink in his restaurant. But, unfortunately, these are not labelled. He has to serve the drinks on the demand of customers. One customer wants acidic drink, another wants basic and third one wants neutral drink. How will Dorji decide which drink is to be served to whom?
Give the names and formulae of two strong acids and two weak acids.
Which chemical is injected into the skin of a person during the nettle leaf hair sting?
How can the effect of these stings be neutralised?
It has been found that rubbing vinegar on the stung area of the skin of a person gives him relief. The person has been stung by:
(a) wasp
(b) ant
(c) honey bee
(d) nettle leaf hair
Name an acid used to flavor and preserve food.
Do basic solutions also have H+(aq)? Why are they basic?
Which acid is used for getting chloride salt?
Two acids ‘A’ and ‘B’ are given. Acid A gives one hydrogen ion per molecule of the acid in solution. Acid B gives two hydrogen ions per molecule of the acid in solution.
- Find out acid A and acid B.
- Which acid is called the King of Chemicals?
Fill in the crossword given in Figure 5.2 with the help of the clues provided.
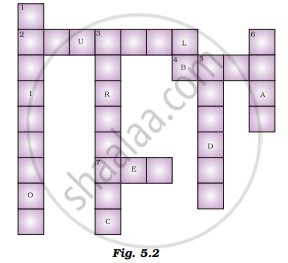
Across
(2) The solution which does not change the colour of either red or blue litmus.
(4) Phenolphthalein gives pink colour in this type of solution.
(7) Colour of blue litmus in lemon juice.
Down
(1) It is used to test whether a substance is acidic or basic.
(3) It is a natural indicator and gives pink colour to the basic solution.
(5) Nature of ant’s sting.
(6) It is responsible for the increase in temperature during a neutralisation reaction.
Name the acid present in the given table.
