Advertisements
Advertisements
Question
Explain Bredig’s arc method.
Solution
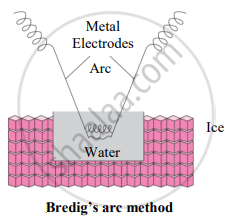
- Colloidal sols can be prepared by electrical disintegration using Bredig’s arc method.
- This process involves vaporization as well as condensation.
- Colloidal sols of metals such as gold, silver, platinum can be prepared by this method.
- In this method, an electric arc is struck between electrodes of metal immersed in the dispersion medium.
- The intense heat produced vapourizes the metal which then condenses to form particles of colloidal sol.
APPEARS IN
RELATED QUESTIONS
Write one difference in Multimolecular colloid and Associated colloid
Write a note on the Tyndall effect.
Write a note on Brownian motion.
What happens when a beam of light is passed through a colloidal sol.
Which of the following is multimolecular colloid?
Identify the CORRECT statements from the following.
i. The colour of colloidal dispersion depends on size of colloidal particles.
ii. Tyndall effect is used to distinguish between colloidal dispersion and true solution.
iii. Eosin and congo red are examples of negatively charged sols.
The order of coagulating power of following ions in the coagulation of a positive sol is:
i. \[\ce{PO^{3-}_4}\]
ii. \[\ce{SO^{2-}_4}\]
iii. \[\ce{[Fe(CN)6]^{4-}}\]
iv. \[\ce{NO^-_3}\]
Tyndall effect is observed due to ____________.
Froth is a colloidal solution of ____________.
Which of the following is multimqlecular colloid?
Some colloids are stable by their nature, i.e., gels, alloys, and solid foams. Gelatin and jellies are two common examples of a gel. The solid and liquid phases in a gel are interdispersed with both phases being continuous. In most systems, the major factor influencing the stability is the charge on the colloidal particles. If a particular ion is preferentially adsorbed on the surface of the particles, the particles in suspension will repel each other, thereby preventing the formation of aggregates that are larger than colloidal dimensions. The ion can be either positive or negative depending on the particular colloidal system, i.e., air bubbles accumulate negative ions, sulphur particles have a net negative charge in a sulphur sol, and the particles in a metal hydroxide sol are positively charged. Accumulation of charge on a surface is not an unusual phenomenon-dust is attracted to furniture surfaces by electrostatic forces. When salts are added to lyophobic colloidal systems the colloidal particles begin to form larger aggregates and a sediment forms as they settle. This phenomenon is called flocculation, and the suspension can be referred to as flocculated, or colloidally unstable. If the salt is removed, the suspension can usually be restored to its original state; this process is called deflocculation or peptization. The original and restored colloidal systems are called deflocculated, peptized, or stable sols.
Why does a small amount of salt have such a dramatic effect on the stability of a lyophobic colloidal system? The answer lies in an understanding of the attractive and repulsive forces that exist between colloidal particles. Van der Waals forces are responsible for the attractions, while the repulsive forces are due to the surface charge on the particles. In a stable colloid, the repulsive forces are of greater magnitude than the attractive forces. The magnitude of the electrical repulsion is diminished by addition of ionized salt, which allows the dispersed particles to aggregate and flocculate. River deltas provide an example of this behaviour. A delta is formed at the mouth of a river because the colloidal clay particles are flocculated when the freshwater mixes with the salt water of the ocean.
Gelatin is a _________ colloidal system.
Which of the following substances will precipitate the negatively charged emulsions?
(i) \[\ce{KCl}\]
(ii) glucose
(iii) urea
(iv) \[\ce{NaCl}\]
Why are some medicines more effective in the colloidal form?
How does the precipitation of colloidal smoke take place in Cottrell precipitator?
Why is \[\ce{Fe(OH)3}\] colloid positively charged, when prepared by adding \[\ce{FeCl3}\] to hot water?
Match the items of Column I and Column II.
| Column I | Column II |
| (i) Butter | (a) dispersion of liquid in liquid |
| (ii) Pumice stone | (b) dispersion of solid in liquid |
| (iii) Milk | (c) dispersion of gas in solid |
| (iv) Paints | (d) dispersion of liquid in solid |
Cloud is an example of
The coagulation of 200 ML of position sol took place when 0.73 HCL was added to its without changing the volume much. The flocculation value of HCL for the colloid is.
Fog is a colloidal solution of ______.
The size of a raw mango shrinks to a much smaller size when kept in a concentrated salt solution. Which one of the following processes can explain this?
The migration of dispersion medium under the influence of an electric potential is called ______.
Which of the following is most powerful to coagulate the negative colloid?
Smoke is an example of ______.
Blood may be purified by ______.
Van Arkel's method of purification of metals involves converting the metal to a ______.
Starch is an example of which of the following type of colloid?
