Advertisements
Advertisements
Question
Explain Maxwell distribution of molecular speed with necessary graph.
Solution
Gas is collection of tiny particles separated from one another by large empty spaces and moving randomly in all directions. In the inter course of their motion, they collide with one another and also with the walls of the container. Due to these collisions, the speed and the direction of motion of the molecules keep on changing. Although. it is not possible to find out the speeds of the individual molecules, yet from the probability considerations it has become possible to work out the distribution of the molecules.
This distribution is referred to as Maxwell-Boltzmann distribution
The distribution of speeds remains fairly constant at a particular temperature althought the individual speeds of the molecules can change.
Maxwell plotted the fraction of molecules having different speed against the speeds at particular temperature. This plot is shown as follow:
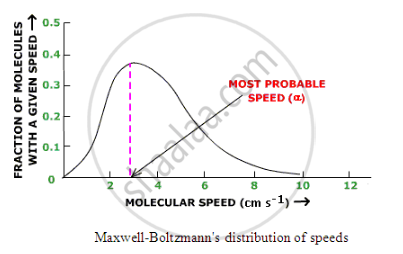
Improtant feature of this curve are:
The fraction of molecules with very low or high speeds is very small
The fraction of molecules possessing higher and higher speeds goes on increasing till it reaches a peak and then starts decreasing
The maximum fraction of molecules possesses a speed, corresponding to the peak in the curve which is referred to as most probable speed
The area under the curve gives the total number of gas molecules.
