Advertisements
Advertisements
Question
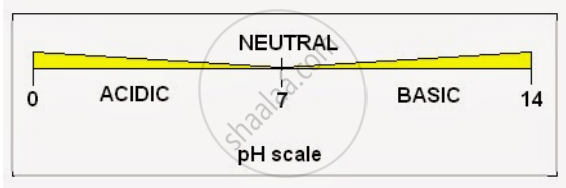
Explain the pH scale with the proper diagram.
Solution
- pH helps in measuring hydrogen ion concentration in solutions. In pH, p stands for “potenz” means “strength” in German.
- The scale reads from O (zero) (most acidic) to 14 (most basic).
- The value of pH indicates the acidic or basic nature of a solution. The strength of the base is represented by pOH.
- When the pH value is between 0 to 7, the solution is acidic in nature. At value 7, the solution is neutral and between 7 to 14 the nature of the solution becomes alkaline/ basic.
- Lower the pH of an acid, greater is the concentration of H+ ions, greater the pH of a base, greater is the concentration of OH ions.
APPEARS IN
RELATED QUESTIONS
Draw neat and labelled diagram of pH scale.
The pH of rainwater is 7.
Which one of the following types of medicines is used for treating indigestion?
The colour of universal indicator solution is ____________.
(a) red
(b) blue
(c) green
(d) greenish yellow
Fill in the blank
Magnesia is used in making __________.
Answer the following:
If pH value of solution ‘A’ is 8, pH value of solution ‘B’ is 7 and pH value of solution ‘C’ is 5.5 then:
A solution 'X' gives orange colour when a drop of it falls on pH paper, while another solution 'Y' gives bluish colour when a drop of it falls on pH paper. What is the nature of both solutions? Determine the pH of solutions 'X' and 'Y'.
Tomato is a natural source of which acid?
Tooth enamel is made up of ____________.
Rain is called acid rain when it:
When copper oxide and dilute hydrochloric acid react, colour changes to ____________.
Which of the following are present in a dilute aqueous solution of hydrochloric acid?
pH of H2O is ____________.
Ag2S reacts with H2SO4 to form ____________.
Assertion: Carbonic acid is a weak acid.
Reason: It ionised completely in an aqueous solution.
Frothing in Yamuna:
The primary reason behind the formation of the toxic foam is the high phosphate content in the wastewater because of detergents used in dyeing industries, dhobi ghats, and households. Yamuna's pollution level is so bad that parts of it have been labelled 'dead' as there is no oxygen in it for aquatic life to survive.

Predict the pH value of the water of river Yamuna if the reason for froth is the high content of detergents dissolved in it.
The table provides the pH value of four solutions P, Q, R and S.
| Solution | pH value |
| P | 2 |
| Q | 9 |
| R | 5 |
| S | 11 |
Which of the following correctly represents the solutions in increasing order of their hydronium ion concentration?
Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper. What would be the colour obtained?

Explain the role of pH in our digestive system.
The pH of stomach fluid is ______.
The pH value of acids is ______ than 7.
The white enamel coating of our teeth is ______.
pH value of human blood is ______.
You are given pure water to test the pH value using pH ______ paper. It shows colour.
Define indicator. Give examples
Correct the wrong statement given below:
The pH value of the base is lesser than 7.
There are four solutions A, B, C and D with pH values as follow:
| Solution | A | B | C | D |
| pH | 2.0 | 7.0 | 8.0 | 12.0 |
Which solution(s) would liberate hydrogen gas with zinc?
Acid present in tomato is ______.
How does Paramecium obtain is food?
List the role of each of the following in our digestive system:
- Hydrochloric acid
- Trypsin
- Muscular walls of stomach
- Salivary amylase
Mohan has three solutions P, Q and R having a pH of 13, 5 and 2 respectively. Which of the above solutions P, Q or R will react with magnesium to liberate hydrogen gas?
Mohan has three solutions P, Q and R having a pH of 13, 5 and 2 respectively. Which of the above solutions P, Q or R will liberate ammonia gas when it reacts with ammonium chloride?
