Advertisements
Advertisements
Question
Explain Schottky defect.
Solution
Schottky defect arises due to the missing of an equal number of cations and anions from the crystal lattice. This effect does not change the stoichiometry of the crystal. Ionic solids in which the cation and anion are of almost of similar size show Schottky defect.
Example: NaCl
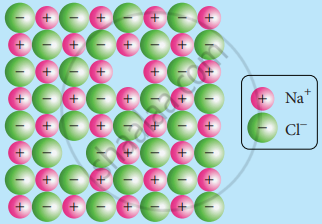
Schottky Defect
Presence of a large number of Schottky defects in a crystal lowers its density. For example, the theoretical density of vanadium monoxide (VO) calculated using the edge length of the unit cell is 6.5 g cm−3 but the actual experimental density is 5.6 g cm−3. It indicates that there is approximately 14% Schottky defect in VO crystal. Presence of the Schottky defect in the crystal provides a simple way by which atoms or ions can move within the crystal lattice.
APPEARS IN
RELATED QUESTIONS
Schottky defect in a crystal is observed when
The cation leaves its normal position in the crystal and moves to some interstitial position, the defect in the crystal is known as ____________.
Assertion: due to Frenkel defect, density of the crystalline solid decreases.
Reason: in Frenkel defect cation and anion leaves the crystal.
The crystal with a metal deficiency defect is ____________.
What are point defects?
Write short note on metal excess and metal deficiency defect with an example.
Write a note on Frenkel defect.
