Advertisements
Advertisements
Question
Explain the postulates of Bohr’s atomic model.
Solution
The main postulates of Neil’s Bohr are as follows:
- In atoms, electrons revolve around the nucleus in special orbits called discrete orbits or shells or energy levels.
- While the electrons revolve, they do not radiate energy.
- The circular orbits are numbered as 1, 2, 3, 4 or designated as K, L, M, N shells. These numbers are referred as principal quantum numbers (n).
- K shell (n = 1) is closer to the nucleus and is associated with lowest energy. L, M, N are the next higher energy levels. As the distance from the nucleus increases, the energy of the shells also increase.
- The energy of each orbit or shell is a fixed quantity.
- As the distance from the nucleus increases, the size of the orbits also increases.
- The maximum number of electrons that can be accommodated in an energy level is 2n2 (n = quantum number of its orbit).
- When an electron absorbs energy, it jumps from lower energy level to higher energy level.
- When an electron returns from higher energy level to lower energy level, it gives off energy.
Energy levels around the nucleus of an atom: Bohr's model.

APPEARS IN
RELATED QUESTIONS
Draw a sketch of Bohr’s model of an atom with three shells.
What are the various letters used by Bohr to represent electron shells in an atom?
In the figure given alongside
(a) Name the shells denoted by A,B, and C. Which shell has least energy
(b) Name X and state the charge on it
(c) The above sketch is of …………. Model of an atom
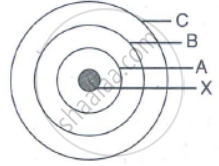
State in brief the drawbacks of Rutherford's atomic model correlating them with the postulates of Bohr’s atomic model.
State true or false. If false, correct the statement.
Smaller the size of the orbit, lower is the energy of the orbit.
Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?
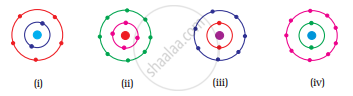
What are the Limitations of Bohr’s Model?
The atomic model based on quantum theory was first proposed by ______.
Bohr's model of atoms ______.
The scientist who proposed the atomic model based on the quantisation of energy for the first time is ______.
