Advertisements
Advertisements
Question
Explain with diagram the process used to – separate the following substance from the given mixtures - Sulphur from a mixture of – sulphur & copper.
Solution
By solvent extraction: Mixture of copper and sulphur is added to the beaker containing solvent carbon disulphide and stirred well. Sulphur dissolves. Put this mixture on filter paper in the funnel. Copper remains on filter paper and sulphur passes into the beaker as filtrate. Sulphur separates as carbon disulphide evaporates.
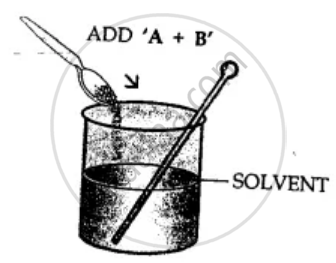
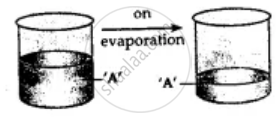
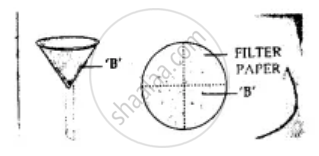
Here A is for Copper and B is Sulphur.
APPEARS IN
RELATED QUESTIONS
Fill in the blan
Iodine, camphor, naphthalene, ammonium chloride and dry ice are some substances that ....................................
How can you separate iron metal from non-magnetic impurities ?
How is distillation method different from evaporation?
Suggest a suitable technique to separate the constituents of the following mixtures. Also give the reason for selecting the particular method.
Ammonium chloride from sand
State any one method – to separate the following mixtures – A liquid-liquid mixture containing – two immiscible'' liquids having different densities
State any one method – to separate the following mixtures – A liquid-gas mixture containing – a gas dissolved in a liquid component.
Explain with diagram the process used to – separate the following substance from the given mixtures - Ammonium chloride from a mixture of – ammonium chloride & potassium chloride.
Give a reason for the following statement :
Zinc is considered an element, while zinc sulphide is considered a compound.
How will you separate sand and water from their mixture?
Grain and husk can be separated with the process of decantation.
