Advertisements
Advertisements
Question
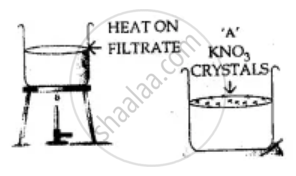
Explain with a diagram the process used to – separate the following substance from the given mixture.
Potassium nitrate from a mixture of – potassium nitrate & potassium chlorate.
Solution
Potassium nitrate KNO3 is more-soluble than potassium chlorate KClO3.
On heating to get the saturated solution and on cooling the saturated solution less soluble (KClO3) crystallise out. More soluble KNO3 is filtered out from the hot saturated solution and is recrystallised from hot water and dried.

APPEARS IN
RELATED QUESTIONS
Write true or false
Handpicking can be used as a separation technique if the particle size of the constituents of the mixture is the same.
Suggest a suitable technique to separate the constituents of the following mixtures. Also give the reason for selecting the particular method.
Ammonium chloride from sand
Suggest a suitable technique to separate the constituents of the following mixtures. Also give the reason for selecting the particular method.
Chalk powder from water
Suggest a suitable technique to separate the constituents of the following mixtures. Also give the reason for selecting the particular method.
Sodium chloride and potassium nitrate
Match the ideal method of separation of components in a mixture in List I with the appropriate process in List II.
| List I | List II |
| 1. Sand from a mixture of sand and water | A: Separating funnel |
| 2. kerosene from a mixture of kerosene water | B: Sublimation |
| 3. Alcohol from a mixture of methyl alcohol and water | C: Filtration |
| 4. Naphthalene from a mixture of naphthalene and lead chloride | D: Distillation |
| 5. Pure water from impure water | E: Fractional distillation |
Compare the properties of iron [II] sulphide with the iron-sulphur mixture, considering iron [II] sulphide as a compound & particles of iron & sulphur mixed together as an example of a mixture.
State any one method – to separate the following mixtures – A liquid-gas mixture containing – a gas dissolved in a liquid component.
Malar’s mother was preparing to cook dinner. She accidentally mixed ground nuts with urad-dhal. Suggest a suitable method to separate the two substances so that Malar can have ground nuts to eat.
A mixture of milk and water can be separated by filtration.
Separation of sugar from tea can be done with filtration.
