Advertisements
Advertisements
Question
Fill in the blanks by choosing the appropriate words from those given in the brackets:
[stable, low, aldehyde, unstable, 6, 4, ethane, Clemmensen’s, 2, 3, carboxylic acid, high, propane, Rosenmund's]
The primary alcohols are easily oxidised first into ______ and then into ______.
Solution
The primary alcohols are easily oxidised first into aldehyde and then into carboxylic acid.
APPEARS IN
RELATED QUESTIONS
Show how the following compound can be converted to benzoic acid.
Phenylethene (Styrene)
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
m-Nitrobenzoic acid
The functional group present in triacylglycerol is _______.
Assertion: Aldehydes and ketones, both react with Tollen’s reagent to form silver mirror.
Reason: Both, aldehydes and ketones contain a carbonyl group.
Substitution of one alkyl group by replacing hydrogen of primary amines
Which of the following substance produced acetaldehyde on dry distillation?
Match List - I with List - II.
| List - I | List - II | ||
| (a) |  \[\ce{->[CO,HCl][Anhyd. AlCl3/CuCl]}\] \[\ce{->[CO,HCl][Anhyd. AlCl3/CuCl]}\] |
(i) | Hell-Volhard-Zelinsky reaction |
| (b) |
\[\begin{array}{cc}
\ce{O}\phantom{.................}\\ ||\phantom{.................}\\ \ce{R - C - CH3 + NaOX ->} \end{array}\] |
(ii) | Gattermann-Koch reaction |
| (c) | \[\ce{R - CH2 - OH + R'COOH ->[Conc. H2SO4]}\] | (iii) | Haloform reaction |
| (d) | \[\ce{R - CH2COOH ->[(i) X2/Red P][(ii) H2O]}\] | (iv) | Esterification |
Choose the correct answer from the options given below.
The end product Y in the sequence of reaction:
\[\ce{RX ->[CN^-] X ->[NaOH] Y}\] is:
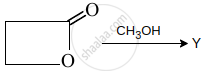
Y is:
Hex-4-ene-2-ol on treatment with PCC gives 'A'. 'A' on reaction with sodium hypoiodite gives 'B', which on further heating with soda lime gives 'C. The compound 'C' is ______.
