Advertisements
Advertisements
Question
For a complex reaction:
(i) order of overall reaction is same as molecularity of the slowest step.
(ii) order of overall reaction is less than the molecularity of the slowest step.
(iii) order of overall reaction is greater than molecularity of the slowest step.
(iv) molecularity of the slowest step is never zero or non interger.
Solution
(i) order of overall reaction is same as molecularity of the slowest step.
(iv) molecularity of the slowest step is never zero or non interger.
Explanation:
(i) For a complex reaction, order of overall reaction = molecularity of slowest step. As rate of overall reaction depends upon total number of molecules involved in slowest step of the reaction. Hence, molecularity of the slowest step is equal to order of overall reaction.
(ii) Since the completion of any chemical reaction is not possible in the absence of reactants. Hence, slowest step of any chemical reaction must contain at least one reactant. Thus, molecularity’of the slowest step is never zero or non-integer.
APPEARS IN
RELATED QUESTIONS
For a reaction A + B ⟶ P, the rate is given by
Rate = k [A] [B]2
How is the rate of reaction affected if the concentration of B is doubled?
Mention the factors that affect the rate of a chemical reaction.
In a reaction between A and B, the initial rate of reaction (r0) was measured for different initial concentrations of A and B as given below:
| A/mol L−1 | 0.20 | 0.20 | 0.40 |
| B/mol L−1 | 0.30 | 0.10 | 0.05 |
| r0/mol L−1 s−1 | 5.07 × 10−5 | 5.07 × 10−5 | 1.43 × 10−4 |
What is the order of the reaction with respect to A and B?
Rate of reaction for the combustion of propane is equal to:
\[\ce{C3H8_{(g)} + 5O2_{(g)} -> 3CO2_{(g)} + 4H2O_{(g)}}\]
Why is the probability of reaction with molecularity higher than three very rare?
Why can’t molecularity of any reaction be equal to zero?
Match the graph given in Column I with the order of reaction given in Column II. More than one item in Column I may link to the same item of Column II.
| Column I | Column II | |
| (i) | 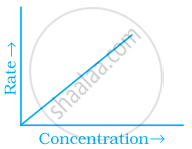 |
|
| (ii) | 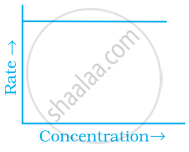 |
(a) 1st order |
| (iii) | 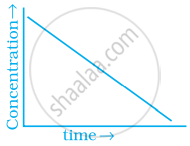 |
(b) Zero-order |
| (iv) | 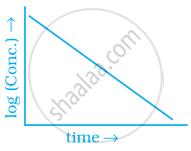 |
The number of molecules of the reactants taking part in a single step of the reaction is indicative of ______.
On heating compound (A) gives a gas (B) which is constituent of air. The gas when treated with H2 in the presence of catalyst gives another gas (C) which is basic in nature, (A) should not be ______.
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. The half-lives for A and B are 300 s and 180 s, respectively. If the concentrations of A and B are equal initially, the time required for the concentration of A to be four times that of B (in s) is ______. (Use ln 2 = 0.693)
