Advertisements
Advertisements
Question
At high pressure the following reaction is zero order.
\[\ce{2NH3(g) ->[1130 K][Platinum catalyst] N2(g) + 3H2(g)}\]
Which of the following options are correct for this reaction?
(i) Rate of reaction = Rate constant.
(ii) Rate of the reaction depends on concentration of ammonia.
(iii) Rate of decomposition of ammonia will remain constant until ammonia disappears completely.
(iv) Further increase in pressure will change the rate of reaction.
Solution
(i) Rate of reaction = Rate constant.
(iii) Rate of decomposition of ammonia will remain constant until ammonia disappears completely.
(iv) Further increase in pressure will change the rate of reaction.
Explanation:
The pressure in this reaction is extremely high and it becomes independent of the ammonia concentration. The metal surface becomes saturated with gas molecules when the rate of reaction = Rate constant. The rate of a zero-order reaction is independent of the concentration of reactants in the reaction.
APPEARS IN
RELATED QUESTIONS
Give one example of zero order reaction.
Which of the following graphs is correct for a zero order reaction?
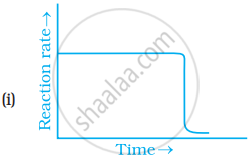
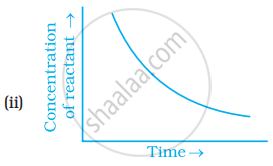
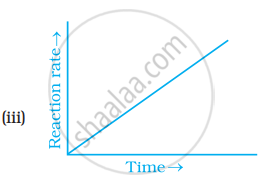

For a zero order reaction will the molecularity be equal to zero? Explain.
A solution with initial concentration of a mol dm-3 follow zero order kinetic. The time taken for the completion of reaction is
Consider the following statement:-
(i) Increase in concentration of reactant increases the rate of a zero-order reaction.
(ii) Rate constant k is equal to collision frequency A if Ea = 0
(iii) Rate constant k is equal to collision frequency A if Ea = 0
(iv) In k vs t is a straight line
(v) In k vs 1/T is a straight line
Which of the above statement is correct?
For a zero-order reaction, the plot of [A]t vs t is linear with a ______
The following experimental rate data were obtained for a reaction carried out at 25°C:
\[\ce{A_{(g)} + B_{(g)} -> C_{(g)} + A_{(g)}}\]
| Initial [A(g)]/mol dm−3 | Initial [B(g)]/mol dm−3 | Initial rate/mol dm−3s−1 |
| 3.0 × 10−2 | 2.0 × 10−2 | 1.89 × 10−4 |
| 3.0 × 10−2 | 4.0 × 10−2 | 1.89 × 10−4 |
| 6.0 × 10−2 | 4.0 × 10−2 | 7.56 × 10−4 |
What are the orders with respect to A(g) and B(g)?
If the initial concentration of substance A is 1.5 M and after 120 seconds the concentration of substance A is 0.75 M, the rate constant for the reaction if it follows zero-order kinetics is ______.
What is zeroth order reaction? Derive its integrated rate Law. What are the units of rate constant?
Write the unit of rate constant of zero order reaction.
