Advertisements
Advertisements
Question
The following experimental rate data were obtained for a reaction carried out at 25°C:
\[\ce{A_{(g)} + B_{(g)} -> C_{(g)} + A_{(g)}}\]
| Initial [A(g)]/mol dm−3 | Initial [B(g)]/mol dm−3 | Initial rate/mol dm−3s−1 |
| 3.0 × 10−2 | 2.0 × 10−2 | 1.89 × 10−4 |
| 3.0 × 10−2 | 4.0 × 10−2 | 1.89 × 10−4 |
| 6.0 × 10−2 | 4.0 × 10−2 | 7.56 × 10−4 |
What are the orders with respect to A(g) and B(g)?
Options
Order with respect to A(g) Order with respect to B(g) Zero Second Order with respect to A(g) Order with respect to B(g) First Zero Order with respect to A(g) Order with respect to B(g) Second Zero Order with respect to A(g) Order with respect to B(g) Second First
Solution
| Order with respect to A(g) | Order with respect to B(g) |
| Second | Zero |
Explanation:
Rate of reaction R = k[A]n [B]m
where n and m are the order of reaction w.r.t
A and B from the given table change A(g), keeping B(g) constant,
R1 = k[3 × 10−2]n [4 × 10−2]m = 1.89 × 10−4
R2 = k[6 × 10−2]n [4 × 10−2]m = 7.56 × 10−4
`(1/2)^"n" = 1/4` ⇒ n = 2
Change B(g), keeping A(g) constant,
R1 = k[3 × 10−2]n [2 × 10−2]m = 1.89 × 10−4
R2 = k[3 × 10−2]n [4 × 10−2]m = 1.89 × 10−4
`(1/2)^"m"` = 1 ⇒ m = 0
Therefore the order of reaction with respect to A(g) and B(g) will be second order and zero order, respectively.
APPEARS IN
RELATED QUESTIONS
The decomposition of NH3 on platinum surface is zero order reaction. What are the rates of production of N2 and H2 if k = 2.5 × 10−4 mol−1 L s−1?
The reaction between A and B is first order with respect to A and zero order with respect to B. Fill in the blanks in the following table:
| Experiment | A/mol L−1 | B/mol L−1 | Initial rate/mol L−1 min−1 |
| I | 0.1 | 0.1 | 2.0 × 10−2 |
| II | ______ | 0.2 | 4.0 × 10−2 |
| III | 0.4 | 0.4 | ______ |
| IV | ______ | 0.2 | 2.0 × 10−2 |
Which of the following graphs is correct for a zero order reaction?
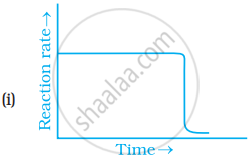
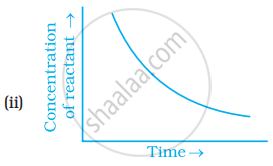
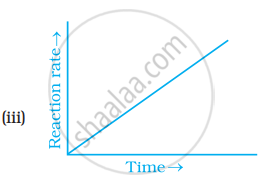

For a zero order reaction will the molecularity be equal to zero? Explain.
For a zero-order reaction, the plot of [A]t vs t is linear with a ______
Write the expression of integrated rate equation for zero order reaction.
The slope in the plot of [R] Vs. time for a zero-order reaction is ______.
If the initial concentration of substance A is 1.5 M and after 120 seconds the concentration of substance A is 0.75 M, the rate constant for the reaction if it follows zero-order kinetics is ______.
What is zeroth order reaction? Derive its integrated rate Law. What are the units of rate constant?
What is zero order reaction?
