Advertisements
Advertisements
Question
Which of the following graphs is correct for a zero order reaction?
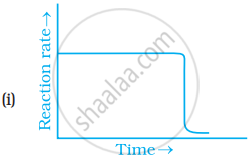
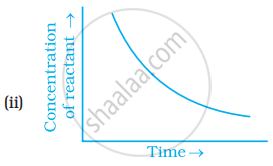
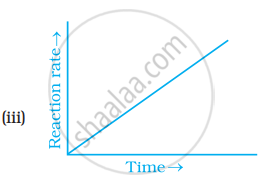

Solution


Explanation:
A Zero order reaction = `[R] = (-k)t + [R]_0`
`y = (m xx x) + c`
x = t(time)
y = [R] concentration
Slope(m) = `- k`
Intercept(c) = `[R]_0`
`([R] - [R]_0)/t = -k`
`([R] - [R]_0)/t = -kt^0`
Rateαt0
APPEARS IN
RELATED QUESTIONS
Give one example of zero order reaction.
For which of the following reaction the units of rate constant and rate of the reaction are same?
At high pressure the following reaction is zero order.
\[\ce{2NH3(g) ->[1130 K][Platinum catalyst] N2(g) + 3H2(g)}\]
Which of the following options are correct for this reaction?
(i) Rate of reaction = Rate constant.
(ii) Rate of the reaction depends on concentration of ammonia.
(iii) Rate of decomposition of ammonia will remain constant until ammonia disappears completely.
(iv) Further increase in pressure will change the rate of reaction.
Write the rate equation for the reaction `2A + B -> C` if the order of the reaction is zero.
For a zero order reaction will the molecularity be equal to zero? Explain.
For a zero-order reaction, the plot of [A]t vs t is linear with a ______
The following experimental rate data were obtained for a reaction carried out at 25°C:
\[\ce{A_{(g)} + B_{(g)} -> C_{(g)} + A_{(g)}}\]
| Initial [A(g)]/mol dm−3 | Initial [B(g)]/mol dm−3 | Initial rate/mol dm−3s−1 |
| 3.0 × 10−2 | 2.0 × 10−2 | 1.89 × 10−4 |
| 3.0 × 10−2 | 4.0 × 10−2 | 1.89 × 10−4 |
| 6.0 × 10−2 | 4.0 × 10−2 | 7.56 × 10−4 |
What are the orders with respect to A(g) and B(g)?
Assertion (A): For a zero-order reaction, the unit of rate constant and rate of reaction are same.
Reason (R): Rate of reaction for zero order reaction is independent of concentration of reactant.
Write the unit of rate constant of zero order reaction.
If unit of rate constant is mol dm−3s−1, the order of reaction would be ______.
