Advertisements
Advertisements
Question
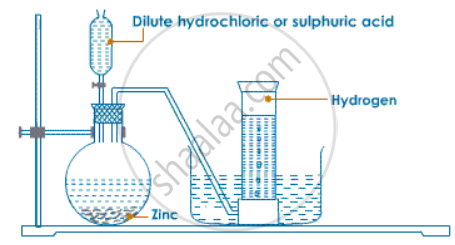
For laboratory preparation of hydrogen, give the following:
fully-labeled diagram
Solution
APPEARS IN
RELATED QUESTIONS
TRUE \ FALSE
Hydrogen does not combine with nitrogen under ordinary conditions.
Give a reason for the following:
Zinc granules are used in the lab preparation of hydrogen.
Complete and balance the following reaction.
Zn + H2O →_____________ +___________
‘Hydrogen is obtained by electrolysis of acidified water’.
Answer the following pertaining to the preparation of hydrogen by electrolysis,
The meaning of the term ‘electrolysis’ and ‘electrolyte’.
Select the correct options for the following statement:
A metal which reacts with water to give a metallic hydroxide & liberate hydrogen.
Select the correct options for the following statement:
A foul smelling gas formed when hydrogen reacts with a molten non-metal, is ___.
Complete and balance the equation:
[General method]
Reactions of metals with dilute acids
Zinc - Zn + 2HCl → ______ + _______ [g]
State the electronic configuration of hydrogen [at. no. 1].e
Give a reason why hydrogen can be placed in group 1 [1A] and group 17 [VIIA] of the periodic table.
Select the correct answer to the reactant added, to give the product in the preparation of hydrogen gas.
\[\ce{Ca(OH)2 + H2}\]
Give a balanced equation for the following conversion.
\[\ce{ZnO<-H2O->Fe3O4}\]
