Advertisements
Advertisements
Question
For the molecule IF7 :
(i) Draw the structure of the molecule.
(ii) State the hybridisation of the central atom.
(iii) State the geometry of the molecule.
Solution
(i)
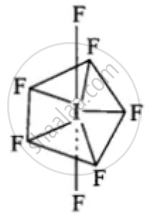
Structure of IF7
(ii) The hybridisation of I is sp3d3
(iii) Geometry is pentagonal bipyramidal.
APPEARS IN
RELATED QUESTIONS
The geometry of XeF6 molecule and_ the hybridization of Xe atom in the molecule.is:
1) Distorted octahedral and `sp^3d^3`
2) Square planar and `sp^3d^2`
3) Pyramidal and `sp^3`
4)Octahedral and `sp^3d^3`
For the molecule XeF2 :
(i) Draw the structure of the molecule indicating the lone pairs.
(ii) State the hybridization of the central atom.
(iii) State the ge.ometry of the molecule.
What are the types of hybridisation of iodine in interhalogen compounds IF3, IF5 and IF7, respectively?
Draw the structure of xenon hexafluoride (XeF6) molecule and state the hybridisation of the central atom.
