Advertisements
Advertisements
Question
Give a balanced equation for the following type of reaction:
A thermal decomposition reaction in which a compound decomposes to give two new compounds.
Solution
| Compound | New compound | New compound | ||
| \[\ce{CaCO3}\] | \[\ce{->[\Delta]}\] | \[\ce{CaO}\] | + | \[\ce{CO2}\] |
| Calcium carbonate | calcium oxide | Carbon dioxide |
APPEARS IN
RELATED QUESTIONS
What does one mean by exothermic reaction? Give example.
What type of reaction is represented by the digestion of food in our body?
What type of chemical reaction take place when electricity is passed through water?
What type of chemical reaction is represented by the following equation?
X → Y + Z
Identify the type of following reaction :
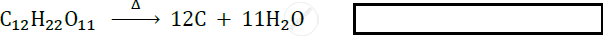
How can the rate of the chemical reaction, namely, decomposition of hydrogen peroxide be increased?
Answer the following question.
2 g of silver chloride is taken in a china dish and the china dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction.
Give the ratio in which hydrogen and oxygen are present in water by volume.
Write the molecular formula of calcium carbonate.
Write one equation for decomposition reactions where energy is supplied in the form of light.
