Advertisements
Advertisements
Question
Give an example of a chemical equation in which two reactants form:
one product
Solution
one product: \[\ce{S + O2->[\triangle]\underset{\text{(sulphur dioxide one product)}}{SO2}}\]
APPEARS IN
RELATED QUESTIONS
How will you indicate Endothermic reaction in a chemical equation?
Balance the given equation:
AI2(SO4)3 +NaOH  AI(OH)3 + Na2SO4
AI(OH)3 + Na2SO4
Giving examples, state the difference between balanced and unbalanced chemical equations.
When potassium nitrate is heated, it decomposes into potassium nitrite and oxygen. Write a balanced equation for this reaction and add the state symbols of the reactants and products.
Balance the following equation:
Zn + KOH → K2ZnO2 + H2
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate the volume of chlorine gas formed at S.T.P.
Match column A with column B.
| Column A | Column B |
| (a) Blue salt changes to white and then black | (i) Ammonium dichromate |
| (b) Orange coloured compound changes to green. | (ii) Iodine |
| (c) Red compound changes to brown and then yellow. | (iii) Zinc Nitrate |
| (d) White to yellow when hot and white when cold. | (iv) Copper sulphate |
| (e) Violet solid changes to violet vapours. | (v) Red Lead |
Balance the following simple equation:
NaOH + Cl2 → NaCl + NaClO + H2O
What are reactants? Explain with the help of example.
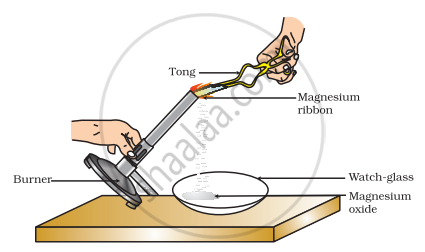
Which of the following is the correct observation of the reaction shown in the above set up?
