Advertisements
Advertisements
Question
Give reason of Ice floats on water.
Solution
Ice has a low density as compared to water. Water has a maximum density at 4°C. That is why ice floats on water.
APPEARS IN
RELATED QUESTIONS
1 kg of ice at 0°C is mixed with 1 kg of steam at 100°C. What will be the composition of the system when thermal equilibrium is reached? Latent heat of fusion of ice = 3.36 × 103 J kg−1 and latent heat of vaporization of water = 2.26 × 106 J kg−1.
What do you mean by the anomalous expansion of water?
Explain the following
A glass bottle completely filled with water and tightly closed at room temperature is likely to burst when kept in the freezer of a refrigerator.
Density of water is maximum at :
What are hot spots? How can you extract energy from a hot spot, if it does not come in contact with underground water?
Why does a thick glass tumbler crack when very hot water is poured in it?
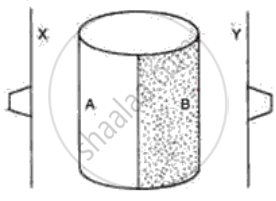
The following figure shows a metal cylinder, containing boiling water. One half side A is polished and another half, B is painted black. Two thin metal sheets X and Y are painted black and have one rubber stopper fixed with wax on each sheet. These sheets are equidistant from the boiling water (container A, B) as shown in the diagram. What would you expect to happen after a few minutes? Give a reason for your answer.
In a region with a cold climate the aquatic animals can survive at 4 °C, because _______.
