Advertisements
Advertisements
Question
Explain the following
A glass bottle completely filled with water and tightly closed at room temperature is likely to burst when kept in the freezer of a refrigerator.
Solution
Inside the freezer, when the temperature of water falls below 4°C, the water in the bottle starts expanding. If the bottle is completely filled and tightly closed, there is no space for water to expand, and hence, the bottle may burst.
APPEARS IN
RELATED QUESTIONS
Explain the following:
How can you relate the formation of water droplets on the outer surface of a bottle taken out of refrigerator with formation of dew?
Answer the following:
On what basis and how will you determine whether air is saturated with vapour or not?
A 50 kg man is running at a speed of 18 km h−1. If all the kinetic energy of the man can be used to increase the temperature of water from 20°C to 30°C, how much water can be heated with this energy?
Describe an experiment to show that water has maximum density at 4°C. What important consequences follow this peculiar property of water? Discuss the importance of this phenomenon in nature.
Explain the following
Fishes survive in ponds even when the atmospheric temperature is well below 0°C.
What do you understand by the anomalous expansion of water?
Why does a thick glass tumbler crack when very hot water is poured in it?
Give reasons for the following:
Even when the water in the lakes is frozen, fish can survive.
While studying anomalous behaviour of water in Hope’s apparatus, the upper temperature of the thermometer : 0 °C : : lower temperature of the thermometer : _______
Observe the given picture and answer the following questions.
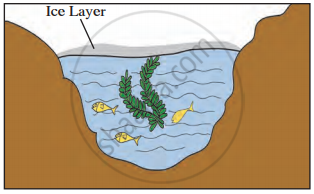
- Which property do you understand in this picture?
- What is the temperature of the water at the surface?
- What is the temperature below the layer of ice on the surface?
