Advertisements
Advertisements
Question
Give reasons for the following:
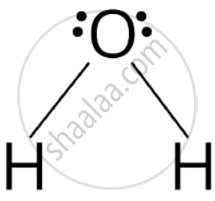
Water molecule has bent structure whereas carbon dioxide molecule is linear.
Short Note
Solution
Due to presence of two lone pairs of electrons on oxygen atom in \[\ce{HiO}\] the repulsion between Ip-lp is more. \[\ce{CO2}\] undergoes sp hybridization resulting in linear shape \[\ce{(O = C = O)}\] while \[\ce{H2O}\] undergoes sp3 hybridisation resulting in distorted tetrahedral or bent structure.
shaalaa.com
Is there an error in this question or solution?
Chapter 4: Chemical Bonding and Molecular Structure - Multiple Choice Questions (Type - I) [Page 45]
