Advertisements
Advertisements
Question
Give the chemical formula of sodium nitrate
Solution
It denotes in a compound, the number of atoms of element present.
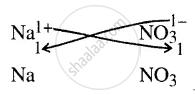
∴ Formula = \[\ce{NaNO3}\]
APPEARS IN
RELATED QUESTIONS
Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
How does the metallic character of elements in Modern Periodic Table vary on moving from left to right in a period, and (ii) down a group?Give reason to justify your answer.
State modern periodic law.
What happens to the number of valence electrons in the atoms of elements as we go down in a group of the periodic table?
Give the symbol and valency of the following element and radical.
Nitrogen [Nitride]
Give the chemical formula of carbonic acid
State whether true or false. If false, correct the statement.
Lanthanides and actinides are kept at the bottom of the periodic table because they resemble each other but they do not resemble with any other group elements.
Which of the given elements A, B, C, D and E with atomic number 2, 3, 7, 10 and 30 respectively belong to the same period?
The element with atomic number 14 is hard and forms acidic oxide and a covalent halide. To which of the following categories does the element belong?
An element X which is a yellow solid at room temperature shows catenation and allotropy. X forms two oxides which are also formed during the thermal decomposition of ferrous sulphate crystals and are the major air pollutants.
- Identify the element X
- Write the electronic configuration of X
- Write the balanced chemical equation for the thermal decomposition of ferrous sulphate crystals?
- What would be the nature (acidic/ basic) of oxides formed?
- Locate the position of the element in the Modern Periodic Table.
