Advertisements
Advertisements
Question
Heat capacity of a body is the ______.
Options
energy needed to melt a body without change in its temperature
energy needed to raise the temperature of a body by 1°C
increase in volume of the body when its temperature increases by 1°C
total amount of internal energy that is constant
Solution
Heat capacity of a body is the energy needed to raise the temperature of a body by 1°C.
APPEARS IN
RELATED QUESTIONS
What is the principle of the method of mixtures?
Define the term heat capacity.
The S.I. unit of heat capacity is ______.
Convection is the process by which the thermal energy flows in solids.
Define thermal capacity.
Metals like copper and aluminum are good conductors of heat and electricity.
The amount of heat required to raise the temperature to 1°C is called ______.
Compare heat capacity and specific heat capacity.
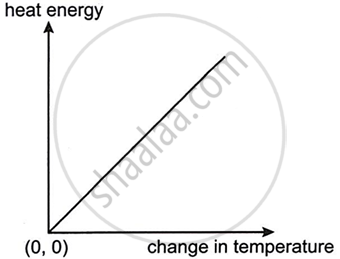
The graph given below shows heat energy supplied against change in temperature when no energy is lost to the surroundings. The slope of this graph will give:
A solid of mass 60 g at 100°C is placed in 150 g of water at 20°C. The final steady temperature is 25°C. Calculate the heat capacity of solid.
[sp. heat capacity of water = 4.2 J g-1 K-1]
