Advertisements
Advertisements
Question
How will you convert benzene into acetophenone?
Solution
Benzene can be converted into acetophenone as:
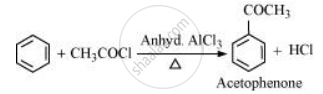
APPEARS IN
RELATED QUESTIONS
How will you convert benzene into m-nitrochlorobenzene?
How will you convert benzene into p -nitrotoluene?
Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Some oxidation reactions of methane are given below. Which of them is/are controlled oxidation reactions?
(i) \[\ce{CH4 (g) + 2O2 (g) -> CO2 (g) + 2H2O (l)}\]
(ii) \[\ce{CH4 (g) + O2 (g) -> C (s) + 2H2O (l)}\]
(iii) \[\ce{CH4 (g) + O2 (g) ->[Mo2O3] HCHO + H2O}\]
(iv) \[\ce{2CH4 (g) + O2 (g) ->[Cu/523 /100 atm] 2CH3OH}\]
Match the reactions given in Column I with the reaction types in Column II.
| Column I | Column II |
| (i) \[\ce{CH2 = CH2 + H2O ->[H+] CH3CH2OH}\] | (a) Hydrogenation |
| (i) \[\ce{CH2 = CH2 + H2 ->[Pd] CH3 - CH3}\] | (b) Halogenation |
| (i) \[\ce{CH2 = CH2 + Cl2 -> Cl - CH2 - CH2 - Cl}\] | (c) Polymerisation |
| (i) \[\ce{3CH ≡ CH ->[Cu tube][Heat] C6H6}\] | (d) Hydration |
| (e) Condensation |
In the halogenation reaction of alkanes, the order of reactivity of different hydrogen in alkanes follows the order:
An alkene ‘A’ contains three \[\ce{C - C}\], eight \[\ce{C - H}\] σ bonds and one \[\ce{C - C}\] π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
An alkene ‘A’ contains three C–C, eight C–H σ bonds and one C–C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
