Advertisements
Advertisements
Question
Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Solution
Addition of HBr to propene is an example of an electrophilic substitution reaction. Hydrogen bromide provides an electrophile, H+. This electrophile attacks the double bond to form 1° and 2° carbocations as shown:
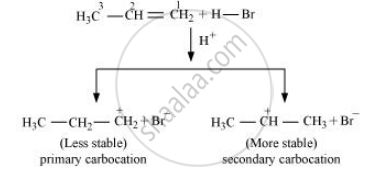
Secondary carbocations are more stable than primary carbocations. Hence, the former predominates since it will form at a faster rate. Thus, in the next step, Br–attacks the carbocation to form 2 – bromopropane as the major product.
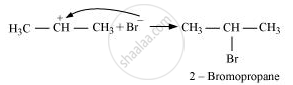
This reaction follows Markovnikov’s rule where the negative part of the addendum is attached to the carbon atom having a lesser number of hydrogen atoms.
In the presence of benzoyl peroxide, an addition reaction takes place anti to Markovnikov’s rule. The reaction follows a free radical chain mechanism as:

Secondary free radicals are more stable than primary radicals. Hence, the former predominates since it forms at a faster rate. Thus, 1 – bromopropane is obtained as the major product.
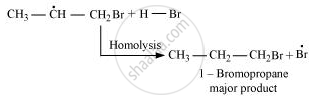
In the presence of peroxide, Br free radical acts as an electrophile. Hence, two different products are obtained on addition of HBr to propene in the absence and presence of peroxide.
APPEARS IN
RELATED QUESTIONS
How will you convert benzene into m-nitrochlorobenzene?
How will you convert benzene into p -nitrotoluene?
How will you convert benzene into acetophenone?
Some oxidation reactions of methane are given below. Which of them is/are controlled oxidation reactions?
(i) \[\ce{CH4 (g) + 2O2 (g) -> CO2 (g) + 2H2O (l)}\]
(ii) \[\ce{CH4 (g) + O2 (g) -> C (s) + 2H2O (l)}\]
(iii) \[\ce{CH4 (g) + O2 (g) ->[Mo2O3] HCHO + H2O}\]
(iv) \[\ce{2CH4 (g) + O2 (g) ->[Cu/523 /100 atm] 2CH3OH}\]
Match the reactions given in Column I with the reaction types in Column II.
| Column I | Column II |
| (i) \[\ce{CH2 = CH2 + H2O ->[H+] CH3CH2OH}\] | (a) Hydrogenation |
| (i) \[\ce{CH2 = CH2 + H2 ->[Pd] CH3 - CH3}\] | (b) Halogenation |
| (i) \[\ce{CH2 = CH2 + Cl2 -> Cl - CH2 - CH2 - Cl}\] | (c) Polymerisation |
| (i) \[\ce{3CH ≡ CH ->[Cu tube][Heat] C6H6}\] | (d) Hydration |
| (e) Condensation |
In the halogenation reaction of alkanes, the order of reactivity of different hydrogen in alkanes follows the order:
An alkene ‘A’ contains three \[\ce{C - C}\], eight \[\ce{C - H}\] σ bonds and one \[\ce{C - C}\] π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
An alkene ‘A’ contains three C–C, eight C–H σ bonds and one C–C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
