Advertisements
Advertisements
Question
What effect does branching of an alkane chain has on its boiling point?
Solution
Alkanes experience inter-molecular Van der Waals forces. The stronger the force, the greater will be the boiling point of the alkane.
As branching increases, the surface area of the molecule decreases which results in a small area of contact. As a result, the Van der Waals force also decreases which can be overcome at a relatively lower temperature. Hence, the boiling point of an alkane chain decreases with an increase in branching.
APPEARS IN
RELATED QUESTIONS
Write IUPAC name of the product obtained by the ozonolysis of the following compound:
3,4-Dimethyl-hept-3-ene
What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
\[\begin{array}{cc}
\ce{D}\phantom{......}\ce{H}\\
\backslash\phantom{......}/\\
\ce{C = C}\\
\phantom{...}/\phantom{......}\backslash\phantom{...}\\\ce{H}\phantom{.......}\ce{D}
\end{array}\]
\[\begin{array}{cc}
\ce{D}\phantom{......}\ce{D}\\
\backslash\phantom{......}/\\
\ce{C = C}\\
\phantom{...}/\phantom{......}\backslash\phantom{...}\\\ce{H}\phantom{.......}\ce{H}\end{array}\]
Find out the type of isomerism exhibited by the following pair.
CH3 – CH2 – NH – CH2 - CH3 and CH3 - NH - CH2 - CH2 - CH3
Find out the type of isomerism exhibited by the following pair.

Molecular formula of the functional isomer of methyl formate is ____________.
But-1-ene and But-2-ene are examples of ____________.
What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?

Which of the following pairs are position isomers?
| I. | \[\begin{array}{cc} \phantom{.......................}\ce{O}\\ \phantom{.......................}||\\ \ce{CH3 - CH2 - CH2 - CH2 - C - H} \end{array}\] |
| II. | \[\begin{array}{cc} \phantom{.................}\ce{O}\\ \phantom{.................}||\\ \ce{CH3 - CH2 - CH2 - C - H} \end{array}\] |
| III. | \[\begin{array}{cc} \ce{CH3 - CH2 - C - CH2 - CH3}\\ \phantom{}||\\ \phantom{}\ce{O} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - C - H}\\ \phantom{...}|\phantom{............}||\phantom{}\\ \phantom{...}\ce{CH3}\phantom{.........}\ce{O}\phantom{} \end{array}\] |
(i) I and II
(ii) II and III
(iii) II and IV
(iv) III and IV
Which of the following pairs are not functional group isomers?
| I. | \[\begin{array}{cc} \phantom{.......................}\ce{O}\\ \phantom{.......................}||\\ \ce{CH3 - CH2 - CH2 - CH2 - C - H} \end{array}\] |
| II. | \[\begin{array}{cc} \phantom{.................}\ce{O}\\ \phantom{.................}||\\ \ce{CH3 - CH2 - CH2 - C - H} \end{array}\] |
| III. | \[\begin{array}{cc} \ce{CH3 - CH2 - C - CH2 - CH3}\\ \phantom{}||\\ \phantom{}\ce{O} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - C - H}\\ \phantom{...}|\phantom{............}||\phantom{}\\ \phantom{...}\ce{CH3}\phantom{.........}\ce{O}\phantom{} \end{array}\] |
(i) II and III
(ii) II and IV
(iii) I and IV
(iv) I and II
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds that represents position isomerism.
Assertion (A): Pent- 1- ene and pent- 2- ene are position isomers.
Reason (R): Position isomers differ in the position of functional group or a substituent.
The molecules having dipole moment are:
(i) 2,2-Dimethylpropane
(ii) trans-Pent-2-ene
(iii) cis-Hex-3-ene
(iv) 2, 2, 3, 3 - Tetramethylbutane.
Assertion (A): The compound cyclooctane has the following structural formula: ![]()
It is cyclic and has conjugated 8π-electron system but it is not an aromatic compound.
Reason (R): (4n + 2)π electrons rule does not hold good and ring is not planar.
Which of the following does NOT exhibit geometrical isomerism?
Ether and alcohol are ______.
The compound which shows metamerism is ______
Which type of isomerism can not be shown by benzaldoxime?
Acetamide is isomer of ______.
Which one of the following pairs are called position isomers?
The correct stereochemical name of
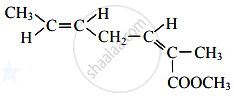
How many structural isomers possible of the molecular formula C3H6O (excluding enol form)?
The number of acyclic structural isomers (including geometrical isomers) for pentene are ______.
Which of the following reactions will not produce a racemic product?
The number of geometrical isomers from [Co(NH3)3(NO2)3] is ______.
The total number of possible isomers of the complex compound [CuII(NH3)4][PtIICl4] is ______.
