Advertisements
Advertisements
Question
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds that represents position isomerism.
Solution
If two or more compounds differ in the position of substituent, functional group, or multiple bonds but molecular formula is same then these are said to be position isomers as they exhibit position isomerism.
In the given structures, I and II, III and IV, VI and VII are found to be the position isomers.
APPEARS IN
RELATED QUESTIONS
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
\[\begin{array}{cc}
\ce{D}\phantom{......}\ce{H}\\
\backslash\phantom{......}/\\
\ce{C = C}\\
\phantom{...}/\phantom{......}\backslash\phantom{...}\\\ce{H}\phantom{.......}\ce{D}
\end{array}\]
\[\begin{array}{cc}
\ce{D}\phantom{......}\ce{D}\\
\backslash\phantom{......}/\\
\ce{C = C}\\
\phantom{...}/\phantom{......}\backslash\phantom{...}\\\ce{H}\phantom{.......}\ce{H}\end{array}\]
Find out the type of isomerism exhibited by the following pair.
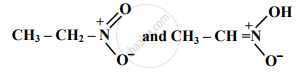
Choose the correct option.
Which type of isomerism is possible in CH3 CHCHCH3?
What type(s) of isomerism is(are) shown by [Co(NH3)4Br2]Cl?
But-1-ene and But-2-ene are examples of ____________.
Which of the following is a functional isomer of pentan-2-ol?
Which of the following pairs are not functional group isomers?
| I. | \[\begin{array}{cc} \phantom{.......................}\ce{O}\\ \phantom{.......................}||\\ \ce{CH3 - CH2 - CH2 - CH2 - C - H} \end{array}\] |
| II. | \[\begin{array}{cc} \phantom{.................}\ce{O}\\ \phantom{.................}||\\ \ce{CH3 - CH2 - CH2 - C - H} \end{array}\] |
| III. | \[\begin{array}{cc} \ce{CH3 - CH2 - C - CH2 - CH3}\\ \phantom{}||\\ \phantom{}\ce{O} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - C - H}\\ \phantom{...}|\phantom{............}||\phantom{}\\ \phantom{...}\ce{CH3}\phantom{.........}\ce{O}\phantom{} \end{array}\] |
(i) II and III
(ii) II and IV
(iii) I and IV
(iv) I and II
Tautomerism is exhibited by ______.
The compound which shows metamerism is ______
Which one of the following pairs are called position isomers?
How many structural isomers possible of the molecular formula C3H6O (excluding enol form)?
The number of acyclic structural isomers (including geometrical isomers) for pentene are ______.
Which of the following pairs of compounds are positional isomers?
The number of geometrical isomers from [Co(NH3)3(NO2)3] is ______.
The total number of possible isomers of the complex compound [CuII(NH3)4][PtIICl4] is ______.
