Advertisements
Advertisements
Question
Choose the correct option.
Which type of isomerism is possible in CH3 CHCHCH3?
Options
Position
Chain
Geometrical
Tautomerism
Solution
Position
APPEARS IN
RELATED QUESTIONS
Write IUPAC name of the product obtained by the ozonolysis of the following compound:
2-Ethylbut-1-ene
What effect does branching of an alkane chain has on its boiling point?
Find out the type of isomerism exhibited by the following pair.
\[\begin{array}{cc}
\ce{CH3 - CH - CH2 - CH3 and CH3 - CH2 - O - CH2 - CH3}\\|\phantom{...........................................}\\
\ce{OH}\phantom{.........................................}\end{array}\]
Molecular formula of the functional isomer of methyl formate is ____________.
What type(s) of isomerism is(are) shown by [Co(NH3)4Br2]Cl?
But-1-ene and But-2-ene are examples of ____________.
What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?

What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
\[\begin{array}{cc}\ce{^+OH}\\||\\
\ce{H - C - OH}\end{array}\]
\[\begin{array}{cc}\ce{OH}\phantom{.}\\|\phantom{...}\\
\ce{H - C^+ - OH}\end{array}\]
In which of the following, functional group isomerism is not possible?
Which of the following pairs are not functional group isomers?
| I. | \[\begin{array}{cc} \phantom{.......................}\ce{O}\\ \phantom{.......................}||\\ \ce{CH3 - CH2 - CH2 - CH2 - C - H} \end{array}\] |
| II. | \[\begin{array}{cc} \phantom{.................}\ce{O}\\ \phantom{.................}||\\ \ce{CH3 - CH2 - CH2 - C - H} \end{array}\] |
| III. | \[\begin{array}{cc} \ce{CH3 - CH2 - C - CH2 - CH3}\\ \phantom{}||\\ \phantom{}\ce{O} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - C - H}\\ \phantom{...}|\phantom{............}||\phantom{}\\ \phantom{...}\ce{CH3}\phantom{.........}\ce{O}\phantom{} \end{array}\] |
(i) II and III
(ii) II and IV
(iii) I and IV
(iv) I and II
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds which are functional group isomers.
Consider structures I to VII and answer the question:
| I. | CH3 – CH2 – CH2 – CH2 – OH |
| II. | \[\begin{array}{cc} \ce{CH3 - CH2 - CH - CH3}\\ \phantom{.....}|\\ \phantom{.......}\ce{OH} \end{array}\] |
| III. | \[\begin{array}{cc} \phantom{...}\ce{CH3}\\ \phantom{}|\\ \ce{CH3 - C - CH3}\\ \phantom{}|\\ \phantom{..}\ce{OH} \end{array}\] |
| IV. | \[\begin{array}{cc} \ce{CH3 - CH - CH2 - OH}\\ |\phantom{........}\\ \ce{CH3}\phantom{......} \end{array}\] |
| V. | CH3 – CH2 – O – CH2 – CH3 |
| VI. | CH3 – O – CH2 – CH2 – CH3 |
| VII. | \[\begin{array}{cc} \ce{CH3 - O - CH - CH3}\\ \phantom{...}|\\ \phantom{......}\ce{CH3} \end{array}\] |
Identify the pairs of compounds that represents chain isomerism.
Compounds with same molecular formula but differing in their structures are said to be structural isomers. What type of structural isomerism is shown by
CH3 – S – CH2 – CH2 – CH3
And
\[\begin{array}{cc}
\phantom{.....................}\ce{CH3}\\
\phantom{................}/\\
\phantom{}\ce{CH3 - S - CH}\\
\phantom{...............}\backslash\\
\phantom{....................}\ce{CH3}
\end{array}\]
The molecules having dipole moment are:
(i) 2,2-Dimethylpropane
(ii) trans-Pent-2-ene
(iii) cis-Hex-3-ene
(iv) 2, 2, 3, 3 - Tetramethylbutane.
Tautomerism is exhibited by ______.
Which type of isomerism can not be shown by benzaldoxime?
Acetamide is isomer of ______.
Which one of the following pairs are called position isomers?
The correct stereochemical name of
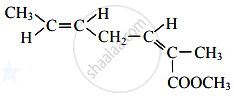
The number of acyclic structural isomers (including geometrical isomers) for pentene are ______.
Compound with molecular formula C3H6O can show ______.
Which of the following pairs of compounds are positional isomers?
The total number of possible isomers of the complex compound [CuII(NH3)4][PtIICl4] is ______.
Which of the following pairs of compounds is an example of position isomerism?
