Advertisements
Online Mock Tests
Chapters
2: Introduction to Analytical Chemistry
3: Basic Analytical Techniques
4: Structure of Atom
5: Chemical Bonding
6: Redox Reactions
7: Modern Periodic Table
8: Elements of Group 1 and 2
9: Elements of Group 13, 14 and 15
10: States of Matter
11: Adsorption and Colloids
12: Chemical Equilibrium
13: Nuclear Chemistry and Radioactivity
▶ 14: Basic Principles of Organic Chemistry
15: Hydrocarbons
16: Chemistry in Everyday Life
![Balbharati solutions for Chemistry [English] 11 Standard chapter 14 - Basic Principles of Organic Chemistry Balbharati solutions for Chemistry [English] 11 Standard chapter 14 - Basic Principles of Organic Chemistry - Shaalaa.com](/images/chemistry-english-11-standard_6:1ddd95908cb04440a83d42566c3337de.jpg)
Advertisements
Solutions for Chapter 14: Basic Principles of Organic Chemistry
Below listed, you can find solutions for Chapter 14 of Maharashtra State Board Balbharati for Chemistry [English] 11 Standard.
Balbharati solutions for Chemistry [English] 11 Standard 14 Basic Principles of Organic Chemistry Exercises [Pages 230 - 232]
Answer the following:
Write condensed formulae and bond line formulae for the following structure.
\[\begin{array}{cc}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\\\phantom{...}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{...}\\\ce{H - C - C - C - C - H}\\|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\\end{array}\]
Write condensed formulae and bond line formulae for the following structure.
\[\begin{array}{cc}\ce{H}\phantom{...}\ce{H}\\|\phantom{....}|\\\ce{N ≡ C - C - C - C ≡ N}\\
|\phantom{....}|\\\ce{H}\phantom{...}\ce{H}\end{array}\]
Write condensed formulae and bond line formulae for the following structure.
\[\begin{array}{cc}
\phantom{.......}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.....}\ce{O}\\
\phantom{.......}|\phantom{....}|\phantom{....}| \phantom{....}//\\
\ce{H - C - C - C - C}\\
\phantom{......}|\phantom{....}|\phantom{....}| \phantom{.....}\backslash\\
\phantom{........}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.....}\ce{OH}
\end{array}\]
Write dash formulae for the following bond line formulae.
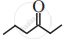
Write dash formulae for the following bond line formulae.
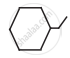
Write dash formulae for the following bond line formulae.
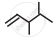
Write dash formulae for the following bond line formulae.

Write bond-line formulae and condensed formulae for the following compound.
3-methyloctane
Write bond-line formulae and condensed formulae for the following compound.
hept-2-ene
Write bond-line formulae and condensed formulae for the following compound.
2, 2, 4, 4-tetramethylpentane
Write bond-line formulae and condensed formulae for the following compound.
octa-1,4-diene
Write bond-line formulae and condensed formulae for the following compound.
Methoxyethane
Write the structural formulae for the following name and also write correct IUPAC names for them.
5-ethyl-3-methylheptane
Write the structural formulae for the following names and also write correct IUPAC names for them.
2,4,5-Trimethylhexane
Write the structural formulae for the following name and also write the correct IUPAC name for it.
2,2,3-trimethylpentan-4-ol
Identify a more favourable resonance structure from the following. Justify.

Identify a more favourable resonance structure from the following. Justify.
\[\begin{array}{cc}
\phantom{.............}\ce{O-}\phantom{......................}\ce{O+}\\\phantom{............}|\phantom{.........................}|\\
\ce{^{+}CH3 - CH = C - H ↔ ^{-}CH2 - CH = C - H}
\end{array}\]
Find out all the functional groups present in the following polyfunctional compound.
Dopamine a neurotransmitter that is deficient in Parkinson's disease.

Find out all the functional groups present in the following polyfunctional compound.
Thyroxine, the principal thyroid hormone.
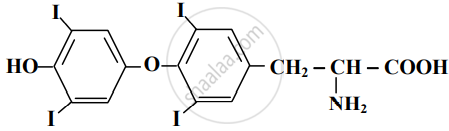
Find out all the functional groups present in the following polyfunctional compound.
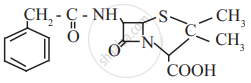
Penicillin G, a naturally occurring antibiotic.
Find out the most stable species from the following. Justify
`dot"CH","CH"_3-dot"CH" - "CH"_3,` \[\begin{array}{cc}\ce{CH3 -\dot{C} - CH3}\\
|\phantom{.}\\\phantom{..}\ce{CH3}\end{array}\]
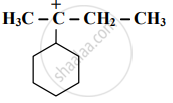
Find out the most stable species from the following. Justify.
`bar"C""H"_3, bar"C""H"_2"Br", bar"C""Br"_3`
Find out the most stable species from the following. Justify.
\[\ce{\overset{+}{C}H3, \overset{+}{C}H2Cl, \overset{+}{C}Cl3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH_3 - CH_2 - \overset{⊕}{C}H-CH_2 - CH_3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
Identify the α-carbon in the following species and give the total number of α-hydrogens.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify primary, secondary, tertiary, and quaternary carbon in the following compound.
\[\begin{array}{cc}\ce{CH3}\phantom{..................}\\
|\phantom{....................}\\\ce{CH3 - C - CH - CH2 - CH2 - CH3}\\|\phantom{.....}|\phantom{................}\\
\ce{CH3}\phantom{.}\ce{CH3}\phantom{..............}
\end{array}\]
Identify primary, secondary, tertiary and quaternary carbon in the following compound.

Match the pairs.
| Column 'A' | Column 'B' | ||
| i. | Inductive effect | a. | delocalisation of π electrons |
| ii. | Hyperconjugation | b. | displacement of π electrons |
| iii. | Resonance effect | c. | delocalisation of σ electrons |
| d. | displacement of σ electrons | ||
What is meant by homologous series?
Write the first four members of the homologous series that begins with CH3CHO. Also, write down their general molecular formula.
Write the first four members of the homologous series that begins with H-C≡C-H. Also, write down their general molecular formula.
Write IUPAC names of the following.

Write the IUPAC name of the following.
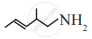
Write the IUPAC name of the following.

Write the IUPAC name of the following.
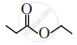
Write the IUPAC name of the following.
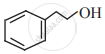
Write the IUPAC name of the following.

Find out the type of isomerism exhibited by the following pair.
CH3 – CH2 – NH – CH2 - CH3 and CH3 - NH - CH2 - CH2 - CH3
Find out the type of isomerism exhibited by the following pair.
\[\begin{array}{cc}
\ce{CH3 - CH - CH2 - CH3 and CH3 - CH2 - O - CH2 - CH3}\\|\phantom{...........................................}\\
\ce{OH}\phantom{.........................................}\end{array}\]
Find out the type of isomerism exhibited by the following pair.
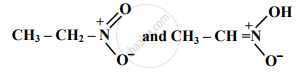
Find out the type of isomerism exhibited by the following pair.

Draw a resonance structure of the following:
Phenol
Draw a resonance structure of the following:
Benzaldehyde
Draw a resonance structure of the following:
Buta-1,3-diene
Draw a resonance structure of the following:
Acetate ion
Distinguish between Inductive effect and resonance effect.
Distinguish between Electrophile and nucleophile.
Distinguish between Carbocation and carbanion.
Distinguish between Homolysis and heterolysis.
Write true or false. Correct the false statement.
Homolytic fission involves the unsymmetrical breaking of a covalent bond.
True
False
Write true or false. Correct the false statement.
Heterolytic fission results in the formation of free radicals.
True
False
Write true or false. Correct the false statement.
Free radicals are negatively charged species.
True
False
Write true or false. Correct the false statement.
Aniline is a heterocyclic compound.
True
False
Phytane is a naturally occurring alkane produced by the alga spirogyra and is a constituent of petroleum. The IUPAC name for phytane is 2,6,10,14-tetramethylhexadecane. Write a zig-zag formula for phytane. How many primary, secondary, tertiary, and quaternary carbons are present in this molecule?
Observe the following structures and answer the questions given below.
- \[\ce{CH3 - CH2 - CH2 - CHO}\]
\[\begin{array}{cc}\ce{CH3 - CH - CHO}\\
|\phantom{...}\\\ce{CH3}\end{array}\]
a. What is the relation between (i) and (ii)?
b. Write IUPAC name of (ii).
c. Draw the functional group isomer of (i).
Observe the following and answer the questions given below:
\[\ce{CH3 - CH3 ->[U.V. light] \overset{\bullet}{C}H3 + \overset{\bullet}{C}H3}\]
- Name the reactive intermediate produced.
- Indicate the movement of electrons by a suitable arrow to produce this intermediate.
- Comment on the stability of this intermediate produced.
An electronic displacement in a covalent bond is represented by the following notation.
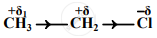
A. Identify the effect
B. Is the displacement of electrons in a covalent bond temporary or permanent.
Draw all the no-bond resonance structures of isopropyl carbocation.
| A covalent bond in tert-butyl bromide breaks in a suitable polar solvent to give ions. |
- Name the anion produced by this breaking of a covalent bond.
- Indicate the type of bond breaking in this case.
- Comment on the geometry of the cation formed by such bond cleavage.
Choose the correct option.
Which of the following statements are true with respect to electronic displacement in a covalent bond?
a. Inductive effect operates through π bond
b. Resonance effect operates through σ bond
c. Inductive effect operates through σ bond
d. Resonance effect operates through π bond
a and b
a and c
c and d
b and c
Choose the correct option.
Hyperconjugation involves overlap of ______ orbitals.
σ - σ
σ -p
p - p
π - π
Choose the correct option.
Which type of isomerism is possible in CH3 CHCHCH3?
Position
Chain
Geometrical
Tautomerism
The correct IUPAC name of the compound 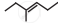 is ______.
is ______.
hept-3-ene
2-ethylpent-2-ene
hex-3-ene
3-methylhex-3-ene
Choose the correct option.
The geometry of a carbocation is ______.
linear
planar
tetrahedral
octahedral
Choose the correct option.
The homologous series of alcohols has general molecular formula ______.
CnH2n+1OH
Cn2n+2OH
CnH2n-2OH
CnH2nOH
Choose the correct option.
The delocalization of electrons due to overlap between p orbital and sigma bond is called _______.
inductive effect
electronic effect
hyperconjugation
resonance
Solutions for 14: Basic Principles of Organic Chemistry
![Balbharati solutions for Chemistry [English] 11 Standard chapter 14 - Basic Principles of Organic Chemistry Balbharati solutions for Chemistry [English] 11 Standard chapter 14 - Basic Principles of Organic Chemistry - Shaalaa.com](/images/chemistry-english-11-standard_6:1ddd95908cb04440a83d42566c3337de.jpg)
Balbharati solutions for Chemistry [English] 11 Standard chapter 14 - Basic Principles of Organic Chemistry
Shaalaa.com has the Maharashtra State Board Mathematics Chemistry [English] 11 Standard Maharashtra State Board solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Balbharati solutions for Mathematics Chemistry [English] 11 Standard Maharashtra State Board 14 (Basic Principles of Organic Chemistry) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Balbharati textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] 11 Standard chapter 14 Basic Principles of Organic Chemistry are Introduction of Basic Principles of Organic Chemistry, Structural Representation of Organic Molecules, Classification of Organic Compounds, Nomenclature of Organic Compounds, Isomerism, Theoretical Basis of Organic Reactions.
Using Balbharati Chemistry [English] 11 Standard solutions Basic Principles of Organic Chemistry exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Balbharati Solutions are essential questions that can be asked in the final exam. Maximum Maharashtra State Board Chemistry [English] 11 Standard students prefer Balbharati Textbook Solutions to score more in exams.
Get the free view of Chapter 14, Basic Principles of Organic Chemistry Chemistry [English] 11 Standard additional questions for Mathematics Chemistry [English] 11 Standard Maharashtra State Board, and you can use Shaalaa.com to keep it handy for your exam preparation.
