Advertisements
Advertisements
Question
Choose the correct option.
The delocalization of electrons due to overlap between p orbital and sigma bond is called _______.
Options
inductive effect
electronic effect
hyperconjugation
resonance
Solution
The delocalization of electrons due to overlap between p orbital and sigma bond is called hyperconjugation.
APPEARS IN
RELATED QUESTIONS
Draw a resonance structure of the following:
Benzaldehyde
Distinguish between Electrophile and nucleophile.
Write true or false. Correct the false statement.
Homolytic fission involves the unsymmetrical breaking of a covalent bond.
Choose the correct option.
Which of the following statements are true with respect to electronic displacement in a covalent bond?
a. Inductive effect operates through π bond
b. Resonance effect operates through σ bond
c. Inductive effect operates through σ bond
d. Resonance effect operates through π bond
Choose the correct option.
Hyperconjugation involves overlap of ______ orbitals.
The correct IUPAC name of the compound 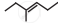 is ______.
is ______.
Choose the correct option.
The homologous series of alcohols has general molecular formula ______.
Which of the following compound is highly reactive towards HCN?
Which of the following is the strongest nucleophile?
Which among the following is a set of nucleophiles?
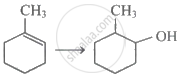
The best reagent for the following conversion is:
Which of the following alkyl groups shows maximum positive inductive effect?
The overlap of σ-p orbitals is called ____________.
Which of the following is the most unstable carbocation?
Which of the following alkyl groups shows least positive inductive effect?
How many tertiary carbon atoms and primary carbon atoms respectively are present in 2-iodo-3, 3- dimethyl pentane?
Which of the following is NOT an electrophile?
How many pi bonds and sigma bonds are present in following molecule?
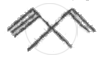
Which of the following statements is not correct?
Identify the functional group that has an electron-donating inductive effect.
Arrange the following free radicals in order of decreasing stability.
- Methyl
- Vinyl
- Allyl
- Benzyl
Identify the α-carbons in the following species and give the total number of α-hydrogen in it.
CH2 = CH − CH2 − CH3
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
