Advertisements
Advertisements
Question
Distinguish between Electrophile and nucleophile.
Solution
| No. | Electrophile | Nucleophile |
| 1. | An electrophile is an electron-deficient species. | A nucleophile is an electron-rich species. |
| 2. | It is attracted towards negative charges (electron seeking). | It is attracted towards a positive charge (nucleus seeking). |
| 3. | It attacks a nucleophilic center in the substrate and brings about an electrophilic reaction | It attacks the electrophilic center in the substrate and brings about a nucleophilic reaction. |
| 4. | It is an electron pair acceptor. (Lewis acid) | It is an electron-pair donor. (Lewis base) |
| 5. | It can be a positively charged ion or a neutral species having a vacant orbital. | It can be negatively charged ion or neutral species having at least one lone pair of electrons |
| 6. | e.g. H+, Br+,\[\ce{NO^+_2}\], BF3, AlCl3, etc. | e.g. OH-, Cl-, CN-, etc. |
APPEARS IN
RELATED QUESTIONS
Identify the α-carbon in the following species and give the total number of α-hydrogens.
\[\ce{CH2 = CH - CH2 - CH3}\]
Draw a resonance structure of the following:
Acetate ion
Distinguish between Homolysis and heterolysis.
Write true or false. Correct the false statement.
Heterolytic fission results in the formation of free radicals.
Write true or false. Correct the false statement.
Aniline is a heterocyclic compound.
Choose the correct option.
Which of the following statements are true with respect to electronic displacement in a covalent bond?
a. Inductive effect operates through π bond
b. Resonance effect operates through σ bond
c. Inductive effect operates through σ bond
d. Resonance effect operates through π bond
Choose the correct option.
The homologous series of alcohols has general molecular formula ______.
Which of the following is NOT an electrophile?
Which of the following is the strongest nucleophile?
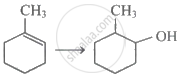
The best reagent for the following conversion is:
The most unstable free radical among the following is:
The overlap of σ-p orbitals is called ____________.
IUPAC name of ![]() is ______.
is ______.
How many tertiary carbon atoms and primary carbon atoms respectively are present in 2-iodo-3, 3- dimethyl pentane?
Which element among the following does form pπ - pπ multiple bonds?
Identify the group that exerts electron withdrawing resonance effect.
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogens.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
