Advertisements
Advertisements
Question
Write true or false. Correct the false statement.
Aniline is a heterocyclic compound.
Options
True
False
Solution
Aniline is a heterocyclic compound.- False
Correct statement:
Aniline is a homocyclic aromatic compound.
APPEARS IN
RELATED QUESTIONS
Find out the most stable species from the following. Justify
`dot"CH","CH"_3-dot"CH" - "CH"_3,` \[\begin{array}{cc}\ce{CH3 -\dot{C} - CH3}\\
|\phantom{.}\\\phantom{..}\ce{CH3}\end{array}\]
Find out the most stable species from the following. Justify.
`bar"C""H"_3, bar"C""H"_2"Br", bar"C""Br"_3`
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH_3 - CH_2 - \overset{⊕}{C}H-CH_2 - CH_3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
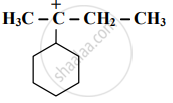
Identify the α-carbon in the following species and give the total number of α-hydrogens.
\[\ce{CH2 = CH - CH2 - CH3}\]
Draw a resonance structure of the following:
Phenol
Distinguish between Inductive effect and resonance effect.
Draw all the no-bond resonance structures of isopropyl carbocation.
Choose the correct option.
Which of the following statements are true with respect to electronic displacement in a covalent bond?
a. Inductive effect operates through π bond
b. Resonance effect operates through σ bond
c. Inductive effect operates through σ bond
d. Resonance effect operates through π bond
The correct IUPAC name of the compound 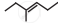 is ______.
is ______.
Which of the following is TRUE for homolytic fission?
Identify the reagent used in the following reaction:
\[\ce{CH3 - CH2 - Br ->[?] CH3 - CH2 - OH}\]
IUPAC name of ![]() is ______.
is ______.
How many tertiary carbon atoms and primary carbon atoms respectively are present in 2-iodo-3, 3- dimethyl pentane?
Resonance is NOT exhibited by ____________.
Which element among the following does form pπ - pπ multiple bonds?
Identify the functional group that has an electron-donating inductive effect.
Identify the α-carbons in the following species and give the total number of α-hydrogen in it.
CH2 = CH − CH2 − CH3
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H -CH2 - CH2 }\]
Identify the α-carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen in each.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H - CH2 - CH3}\]
Identify the α - carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H -CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
