Advertisements
Advertisements
Question
Draw a resonance structure of the following:
Phenol
Solution
Resonance structures for phenol:

APPEARS IN
RELATED QUESTIONS
Find out the most stable species from the following. Justify.
\[\ce{\overset{+}{C}H3, \overset{+}{C}H2Cl, \overset{+}{C}Cl3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
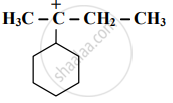
Distinguish between Inductive effect and resonance effect.
Distinguish between Electrophile and nucleophile.
Write true or false. Correct the false statement.
Heterolytic fission results in the formation of free radicals.
Write true or false. Correct the false statement.
Free radicals are negatively charged species.
Choose the correct option.
Which of the following statements are true with respect to electronic displacement in a covalent bond?
a. Inductive effect operates through π bond
b. Resonance effect operates through σ bond
c. Inductive effect operates through σ bond
d. Resonance effect operates through π bond
Choose the correct option.
The delocalization of electrons due to overlap between p orbital and sigma bond is called _______.
Which of the following is TRUE for homolytic fission?
Identify the reagent used in the following reaction:
\[\ce{CH3 - CH2 - Br ->[?] CH3 - CH2 - OH}\]
Which of the following shows positive resonance (+R) effect?
Which of the following alkyl groups shows maximum positive inductive effect?
How many tertiary carbon atoms and primary carbon atoms respectively are present in 2-iodo-3, 3- dimethyl pentane?
Which of the following is NOT an electrophile?
Which element among the following does form pπ - pπ multiple bonds?
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α - carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{⊕}{C}H - CH2 - CH3}\]
Identify the α-carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
CH2 = CH - CH2 - CH3
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
Identify the α - carbon in the following species and give the total number of α-hydrogen.
\[\ce{CH3 - CH2 - \overset{\oplus}{C}H -CH2 - CH3}\]
Identify the α-carbons in the following species and give the total number of α-hydrogen.
\[\ce{CH2 = CH - CH2 - CH3}\]
