Advertisements
Advertisements
Question
Identify a more favourable resonance structure from the following. Justify.

Solution

Structure (I) will be a more favourable resonance structure as structure (II) involves the separation of opposite charges and the electronegative oxygen atom has a positive charge.
APPEARS IN
RELATED QUESTIONS
Match the pairs.
| Column 'A' | Column 'B' | ||
| i. | Inductive effect | a. | delocalisation of π electrons |
| ii. | Hyperconjugation | b. | displacement of π electrons |
| iii. | Resonance effect | c. | delocalisation of σ electrons |
| d. | displacement of σ electrons | ||
Observe the following and answer the questions given below:
\[\ce{CH3 - CH3 ->[U.V. light] \overset{\bullet}{C}H3 + \overset{\bullet}{C}H3}\]
- Name the reactive intermediate produced.
- Indicate the movement of electrons by a suitable arrow to produce this intermediate.
- Comment on the stability of this intermediate produced.
| A covalent bond in tert-butyl bromide breaks in a suitable polar solvent to give ions. |
- Name the anion produced by this breaking of a covalent bond.
- Indicate the type of bond breaking in this case.
- Comment on the geometry of the cation formed by such bond cleavage.
Which one of the following names does not fit a real name?
IUPAC name of \[\begin{array}{cc}
\phantom{....}\ce{H}\phantom{...}\ce{C4H9}\\
|\phantom{....}|\\\ce{CH3 - C - C - CH3}\\
|\phantom{....}|\\\phantom{.....}\ce{C2H5}\phantom{.}\ce{CH3}\phantom{...}\end{array}\] is
The structure of isobutyl group in an organic compound is
Give the IUPAC names of the following compound.
\[\ce{(CH3)2 CH–CH2 –CH(CH3 )–CH(CH3)2}\]
Give the IUPAC names of the following compound.
\[\ce{CH3 - O - CH3}\]
Give the IUPAC names of the following compound.
\[\ce{CH2 = CH - CH = CH2}\]
Give the IUPAC names of the following compound.
\[\begin{array}{cc}\ce{CH3 - C ≡ C - CH - CH3}\\
\phantom{........}|\\\phantom{.........}\ce{Cl}
\end{array}\]
Give the IUPAC names of the following compound.
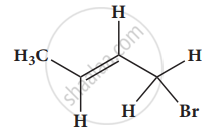
Give the IUPAC names of the following compound.
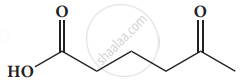
Give the IUPAC names of the following compound.
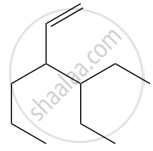
Give the IUPAC names of the following compound.
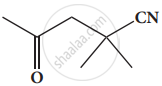
Give the structure for the following compound.
tertiary butyl iodide
Give the structure for the following compound.
3 - Chlorobutanal
Give the structure for the following compound.
2 - Chloro - 2- methyl propane
Give the structure for the following compound.
Butan - 2, 2 - diol
As per IUPAC nomenclature, the name of the complex [Fe(H2O)5(NCS)]2+ is ____________.
What is the IUPAC name of the following compound?
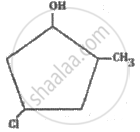
What is a common name of the compound 1-Chloro-2, 2-dimethylpropane?
In the trivial system which prefix will be used for the following compound?
\[\begin{array}{cc}
\ce{CH3}\phantom{.}\\
\phantom{...}\backslash\\
\ce{CH3 - C -}\\
\phantom{...}/\\
\ce{CH3}\phantom{.}\end{array}\]
The IUPAC name of the following compound is:
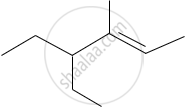
Write the structural formulae for the following name and also write the correct IUPAC name for them.
2,2,3-trimethylpentan-4-ol
