Advertisements
Advertisements
Question
Match the pairs.
| Column 'A' | Column 'B' | ||
| i. | Inductive effect | a. | delocalisation of π electrons |
| ii. | Hyperconjugation | b. | displacement of π electrons |
| iii. | Resonance effect | c. | delocalisation of σ electrons |
| d. | displacement of σ electrons | ||
Solution
| Column 'A' | Column 'B' | ||
| i. | Inductive effect | d. | displacement of σ electrons |
| ii. | Hyperconjugation | c. | delocalisation of σ electrons |
| iii. | Resonance effect | a. | Delocalization of π electrons |
APPEARS IN
RELATED QUESTIONS
Identify a more favourable resonance structure from the following. Justify.

Find out all the functional groups present in the following polyfunctional compound.
Dopamine a neurotransmitter that is deficient in Parkinson's disease.

Write IUPAC names of the following.

Write the IUPAC name of the following.
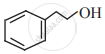
Observe the following structures and answer the questions given below.
- \[\ce{CH3 - CH2 - CH2 - CHO}\]
\[\begin{array}{cc}\ce{CH3 - CH - CHO}\\
|\phantom{...}\\\ce{CH3}\end{array}\]
a. What is the relation between (i) and (ii)?
b. Write IUPAC name of (ii).
c. Draw the functional group isomer of (i).
An electronic displacement in a covalent bond is represented by the following notation.
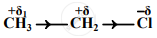
A. Identify the effect
B. Is the displacement of electrons in a covalent bond temporary or permanent.
| A covalent bond in tert-butyl bromide breaks in a suitable polar solvent to give ions. |
- Name the anion produced by this breaking of a covalent bond.
- Indicate the type of bond breaking in this case.
- Comment on the geometry of the cation formed by such bond cleavage.
IUPAC name of \[\begin{array}{cc}
\phantom{....}\ce{H}\phantom{...}\ce{C4H9}\\
|\phantom{....}|\\\ce{CH3 - C - C - CH3}\\
|\phantom{....}|\\\phantom{.....}\ce{C2H5}\phantom{.}\ce{CH3}\phantom{...}\end{array}\] is
The IUPAC name of the compound \[\begin{array}{cc}\ce{CH3 - CH - COOH}\\
|\phantom{.....}\\\ce{OH}\phantom{...}
\end{array}\] is
What is meant by a functional group?
Give the IUPAC names of the following compound.
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
\phantom{...}|\phantom{......}|\phantom{.....}\\
\ce{CH3}\phantom{...}\ce{Br}\phantom{.}
\end{array}\]
Give the IUPAC names of the following compound.
\[\ce{CH3 - O - CH3}\]
Give the IUPAC names of the following compound.
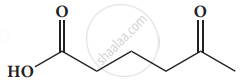
Give the IUPAC names of the following compound.
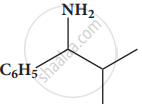
Give the IUPAC names of the following compound.
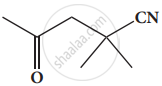
Give the IUPAC names of the following compound.
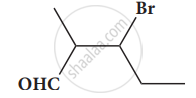
Give the structure for the following compound.
Octane - 1,3- diene
Give the structure for the following compound.
3 - methylbutan - 2 - ol
As per IUPAC nomenclature, the name of the complex Na3[AlF6] is ____________.
In the reaction, \[\ce{Anisole + {'A'} ->[Anhydrous][AlCl3] 4-Methoxyacetophenone}\]
'A' is ____________.
IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3}\phantom{..}\\
|\phantom{....}\\
\ce{CH3 - C - CH2CH3}\\
|\phantom{....}\\
\ce{OH}\phantom{..}
\end{array}\]
What is a common name of the compound 1-Chloro-2, 2-dimethylpropane?
The IUPAC name of the following compound is:
CH2 = C = CH − CH3
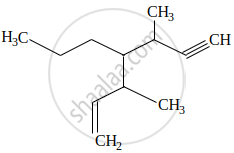
The IUPAC name for the following compound is:
Which one of the following reactions is expected to readily give a hydrocarbon product in good yields?
Write the structural formulae for the following name and also write the correct IUPAC name for them.
2,2,3-trimethylpentan-4-ol
