Advertisements
Advertisements
Question
Observe the following structures and answer the questions given below.
- \[\ce{CH3 - CH2 - CH2 - CHO}\]
\[\begin{array}{cc}\ce{CH3 - CH - CHO}\\
|\phantom{...}\\\ce{CH3}\end{array}\]
a. What is the relation between (i) and (ii)?
b. Write IUPAC name of (ii).
c. Draw the functional group isomer of (i).
Solution
- (a) and (b) are chain isomers of each other.
- IUPAC name of structure (b) is 2-methylpropanal.
- Functional group isomer of (a) is butanone.
\[\begin{array}{cc}\ce{H3C - CH2 - C - CH3}\\
\phantom{.......}||\\\phantom{.......}\ce{\underset{\text{Butanone}}{O}}\end{array}\]
APPEARS IN
RELATED QUESTIONS
Write the IUPAC name of the following.
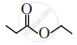
Write the IUPAC name of the following.
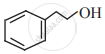
Write the IUPAC name of the following.

Phytane is a naturally occurring alkane produced by the alga spirogyra and is a constituent of petroleum. The IUPAC name for phytane is 2,6,10,14-tetramethylhexadecane. Write a zig-zag formula for phytane. How many primary, secondary, tertiary, and quaternary carbons are present in this molecule?
An electronic displacement in a covalent bond is represented by the following notation.
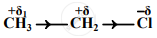
A. Identify the effect
B. Is the displacement of electrons in a covalent bond temporary or permanent.
The IUPAC name of the compound is
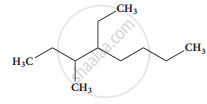
The IUPAC name of \[\begin{array}{cc}
\phantom{.}\ce{CH3}\\|\phantom{..}\\
\ce{H3C - C - CH = C(CH3)2}\\
|\phantom{..}\\\phantom{..}\ce{CH3}
\end{array}\] is
The IUPAC name of the compound\[\begin{array}{cc}\ce{CH3-CH=C-CH2-CH3}\\
|\phantom{..}\\\phantom{...............}\ce{CH2 - CH2 - CH3}\end{array}\] is
The IUPAC name of the compound \[\begin{array}{cc}\ce{CH3 - CH - COOH}\\
|\phantom{.....}\\\ce{OH}\phantom{...}
\end{array}\] is
The IUPAC name of \[\begin{array}{cc}
\ce{CH3}\phantom{.....................}\\
\backslash\phantom{....................}\\
\ce{CH - CH - COOH}\\/\phantom{.......}|\phantom{.............}\\
\ce{Br}\phantom{.......}\ce{CH3}\phantom{...........}\\\end{array}\] is
Give the IUPAC names of the following compound.
\[\ce{CH2 = CH - CH = CH2}\]
Give the IUPAC names of the following compound.
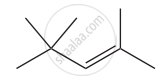
Give the IUPAC names of the following compound.
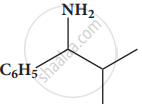
Give the IUPAC names of the following compound.
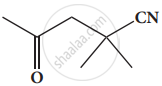
Give the IUPAC names of the following compound.
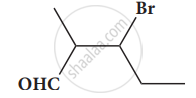
Give the structure for the following compound.
1,3,5- Trimethyl cyclohex - 1 -ene
Give the structure for the following compound.
tertiary butyl iodide
Give the structure for the following compound.
acetaldehyde
Hybridisation of C-atom to which -OH group is attached in the below given compound is:
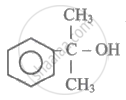
lUP AC name of
\[\begin{array}{cc}
\phantom{}\ce{C2H5}\phantom{.}\ce{Cl}\phantom{....}\ce{CH3}\phantom{......}\\
\phantom{}|\phantom{.....}|\phantom{......}|\phantom{........}\\
\ce{H3C - CH2 - CH - CH - CH - CH2 - CH2 - CH3}
\end{array}\]
IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3}\phantom{..}\\
|\phantom{....}\\
\ce{CH3 - C - CH2CH3}\\
|\phantom{....}\\
\ce{OH}\phantom{..}
\end{array}\]
What is the IUPAC name of the following compound?
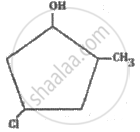
The IUPAC name of the following compound is:
CH2 = C = CH − CH3
The IUPAC name of the following compound is:
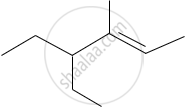
Write the structural formulae for the following name and also write the correct IUPAC names for that.
2,2,3-trimethylpentan-4-ol
