Advertisements
Advertisements
Question
Write the IUPAC name of the following.
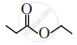
Solution
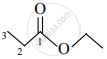
Ethyl propanoate
APPEARS IN
RELATED QUESTIONS
Identify a more favourable resonance structure from the following. Justify.
\[\begin{array}{cc}
\phantom{.............}\ce{O-}\phantom{......................}\ce{O+}\\\phantom{............}|\phantom{.........................}|\\
\ce{^{+}CH3 - CH = C - H ↔ ^{-}CH2 - CH = C - H}
\end{array}\]
Write IUPAC names of the following.

Write the IUPAC name of the following.

Observe the following structures and answer the questions given below.
- \[\ce{CH3 - CH2 - CH2 - CHO}\]
\[\begin{array}{cc}\ce{CH3 - CH - CHO}\\
|\phantom{...}\\\ce{CH3}\end{array}\]
a. What is the relation between (i) and (ii)?
b. Write IUPAC name of (ii).
c. Draw the functional group isomer of (i).
Observe the following and answer the questions given below:
\[\ce{CH3 - CH3 ->[U.V. light] \overset{\bullet}{C}H3 + \overset{\bullet}{C}H3}\]
- Name the reactive intermediate produced.
- Indicate the movement of electrons by a suitable arrow to produce this intermediate.
- Comment on the stability of this intermediate produced.
An electronic displacement in a covalent bond is represented by the following notation.
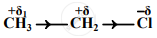
A. Identify the effect
B. Is the displacement of electrons in a covalent bond temporary or permanent.
In the hydrocarbon
![]() the state of hybridisation of carbon 1,2,3,4 and 7 are in the following sequence.
the state of hybridisation of carbon 1,2,3,4 and 7 are in the following sequence.
IUPAC name of \[\begin{array}{cc}
\phantom{....}\ce{H}\phantom{...}\ce{C4H9}\\
|\phantom{....}|\\\ce{CH3 - C - C - CH3}\\
|\phantom{....}|\\\phantom{.....}\ce{C2H5}\phantom{.}\ce{CH3}\phantom{...}\end{array}\] is
The IUPAC name of \[\begin{array}{cc}
\phantom{.}\ce{CH3}\\|\phantom{..}\\
\ce{H3C - C - CH = C(CH3)2}\\
|\phantom{..}\\\phantom{..}\ce{CH3}
\end{array}\] is
The IUPAC name of the compound\[\begin{array}{cc}\ce{CH3-CH=C-CH2-CH3}\\
|\phantom{..}\\\phantom{...............}\ce{CH2 - CH2 - CH3}\end{array}\] is
Give the IUPAC names of the following compound.
\[\begin{array}{cc}
\ce{CH3 - CH - CH - CH3}\\
\phantom{...}|\phantom{......}|\phantom{.....}\\
\ce{CH3}\phantom{...}\ce{Br}\phantom{.}
\end{array}\]
Give the IUPAC names of the following compound.
\[\begin{array}{cc}\ce{CH3 - CH2 - CH - CHO}\\
\phantom{.....}|\\\phantom{.......}\ce{OH}
\end{array}\]
Give the IUPAC names of the following compound.
\[\ce{CH2 = CH - CH = CH2}\]
Give the IUPAC names of the following compound.
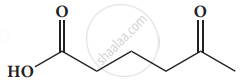
Give the IUPAC names of the following compound.
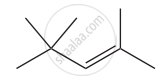
Give the IUPAC names of the following compound.

Give the IUPAC names of the following compound.
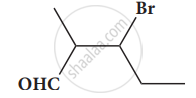
Give the structure for the following compound.
2 - Chloro - 2- methyl propane
Give the structure for the following compound.
3 - methylbut -1- ene
lUP AC name of
\[\begin{array}{cc}
\phantom{}\ce{C2H5}\phantom{.}\ce{Cl}\phantom{....}\ce{CH3}\phantom{......}\\
\phantom{}|\phantom{.....}|\phantom{......}|\phantom{........}\\
\ce{H3C - CH2 - CH - CH - CH - CH2 - CH2 - CH3}
\end{array}\]
In the reaction, \[\ce{Anisole + {'A'} ->[Anhydrous][AlCl3] 4-Methoxyacetophenone}\]
'A' is ____________.
What is a common name of the compound 1-Chloro-2, 2-dimethylpropane?
The correct structure of 2,6-Dimethyl-dec-4-ene is ______
The IUPAC name for the following compound is:
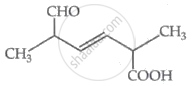
The IUPAC name of the following compound is:
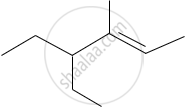
What is the IUPAC name of the compound?
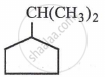
Identify the α-carbons in the following species and give the total number of α-hydrogen.
CH2 = CH - CH2 - CH3
Write the structural formulae for the following name and also write the correct IUPAC names for that.
2,2,3-trimethylpentan-4-ol
Write the structural formulae for the following name and also write the correct IUPAC name for that.
2,2,3-trimethylpentan-4-ol
